Abstract
Objectives
To define the risk of occult cervical metastasis of maxillary squamous cell carcinoma (SCC) and the therapeutic value of elective neck dissection (END) in survival of clinically negative neck node (cN0) patients.
Materials and Methods
Sixty-seven patients with maxillary SCC and cN0 neck were analyzed retrospectively, including 35 patients with maxillary gingiva and 32 patients with maxillary sinus.
Results
Of 67 patients, 10 patients (14.9%) had occult cervical metastasis. The incidence of occult cervical metastasis of maxillary gingival SCC was higher than that of maxillary sinus SCC (17.1% and 12.5%, respectively). The 5-year overall survival rate was 51.9% for the END group and 74.0% for the non-END group. The success rate of treatment for regional recurrence was high at 71.4%, whereas that for local or locoregional recurrence was low (33.3% and 0%, respectively).
Conclusion
The incidence of occult cervical metastasis of maxillary SCC was not high enough to recommend END. For survival of cN0 patients, local control of the primary tumor is more important than modality of neck management. Observation of cN0 neck is recommended when early detection of regional recurrence is possible irrespective of the site or T stage. The key enabler of early detection is patient education with periodic follow-up.
Cervical lymph node metastasis is an important prognostic factor for head and neck cancer patients1234. Neck management for patients with evidence of cervical lymph node metastasis includes consensus-neck dissection and/or neck irradiation. Elective neck dissection (END) for patients with clinically negative neck nodes (cN0), however, remains controversial. There has been no prospective study of the risk of occult cervical metastasis of maxillary squamous cell carcinoma (SCC) because of its low occurrence. Traditionally, observation has been recommended for patients with maxillary SCC and cN0 based on the low risk of cervical metastasis of maxillary SCC. However, several recent retrospective studies have reported that maxillary SCC shows aggressive regional (cervical) metastatic behavior and that risk of occult cervical metastasis is higher than previously supposed5678. In this study, the authors conducted a retrospective analysis of patients with maxillary SCC to define the risk of occult cervical metastasis and the therapeutic value of END for survival of cN0 patients. This study aimed to give recommendations for cN0 neck management based on this analysis.
This study followed the Declaration of Helsinki on medical protocol and ethics, and was approved by the regional Ethical Review Board of the Yonsei Dental Hospital Institutional Review Board (IRB No. 2-2014-0032).
Medical records of patients with maxillary SCC at the Department of Oral and Maxillofacial Surgery, Yonsei University Dental Hospital between 1993 and 2011 were screened. The primary site was categorized as maxillary gingiva or maxillary sinus. Maxillary gingiva indicates mucosa covering the alveolus and palate. A total of 124 patients with maxillary SCC were treated. All patients were examined by computed tomography (CT) and/or magnetic resonance imaging (MRI) to diagnose the primary tumor and cervical lymph nodes. Cancer staging based on the American Joint Committee on Cancer 7th edition TNM staging system9 was conducted. Maxillary sinus SCC and gingival SCC were classified differently according to the TNM staging system. Among them, 27 patients diagnosed with clinically positive neck node (cN+) were excluded. Of 97 patients diagnosed as cN0 neck, cases including any one of the following criteria were excluded (1) patients who had had radiotherapy or chemotherapy before surgery, (2) patients who were not treated surgically, and (3) patients with distant metastasis at initial assessment. Ultimately, 67 patients satisfied the criteria, including 35 patients with maxillary gingiva and 32 patients with maxillary sinus. All patients underwent surgical resection of their primary tumor. The surgeries were performed by 3 oral and maxillofacial surgeons. Each surgeon made the decision on whether END was performed based on tumor site, tumor size, and patient general condition. When conducting END, selective neck dissection with level I-III or modified radical neck dissection on the ipsilateral neck was performed.
The following are definitions of occult cervical metastasis:
In this study, the authors modified the definitions by Poeschl et al.5, who considered only regional recurrence (not locoregional) as occult cervical metastasis. However, the authors added locoregional recurrence to the definitions because regional recurrence could occur independently of local recurrence.
After surgery, patients were followed-up every 3-6 months. MRI or positron emission tomography-CT was used routinely to detect the failure of primary site and neck. When recurrence was evident clinically and radiographically, salvage surgery and/or concurrent chemoradiotherapy was done.
The data between groups were compared by a Mann-Whitney test for continuous variables and a Fisher's exact test for categorical variables. Survival curves were generated using the Kaplan-Meier method and differences between survival curves were compared by the log-rank test. Statistics were considered significant if P<0.05. All statistical analyses were performed with PASW Statistics 18.0 (IBM Co., Armonk, NY, USA).
Of 67 patients satisfying the criteria, 23 were female and 44 were male; mean age was 60.2 years (range, 34-83 years). The mean observation period was 74.8 months (range, 0-216 months). Of 67 patients, 9 patients (13.4%) underwent END simultaneously with primary tumor resection (END group) and 58 patients (86.6%) were treated by primary tumor resection (non-END group).(Table 1) The primary tumor site was maxillary gingiva in 35 patients and maxillary sinus in 32 patients. The rate of maxillary gingiva to maxillary sinus was higher in the END than in the non-END group (77.8% and 48.3%, respectively; P>0.05). That meant there was a tendency to conduct END for a maxillary gingiva primary site, but the difference was not statistically significant. In addition, there was a slight tendency to conduct END for T4 staged tumors (39.7% of non-END group, 55.6% of END group; P>0.05). The distribution of histological grade and clearance of tumor resection margin was similar between the 2 groups.
Table 2 summarizes the patterns of recurrence after primary surgery according to the modality of neck management. Of the 9 patients who underwent END, positive lymph node was confirmed pathologically in one patient. During the follow-up period, one patient in the END group whose neck had been confirmed pathologically negative had locoregional recurrence outside the dissected levels. In the non-END group, locoregional recurrence was detected in one patient and regional recurrence was detected in 7 patients at 12 months on average (range, 1-47 months) after primary surgery. Therefore, 10 of 67 patients had occult cervical metastasis with an incidence of 14.9%. Incidence of occult cervical metastasis for SCC in maxillary gingiva patients was higher than in maxillary sinus patients, being 17.1% (6 of 35 patients) and 12.5% (4 of 32 patients), respectively.
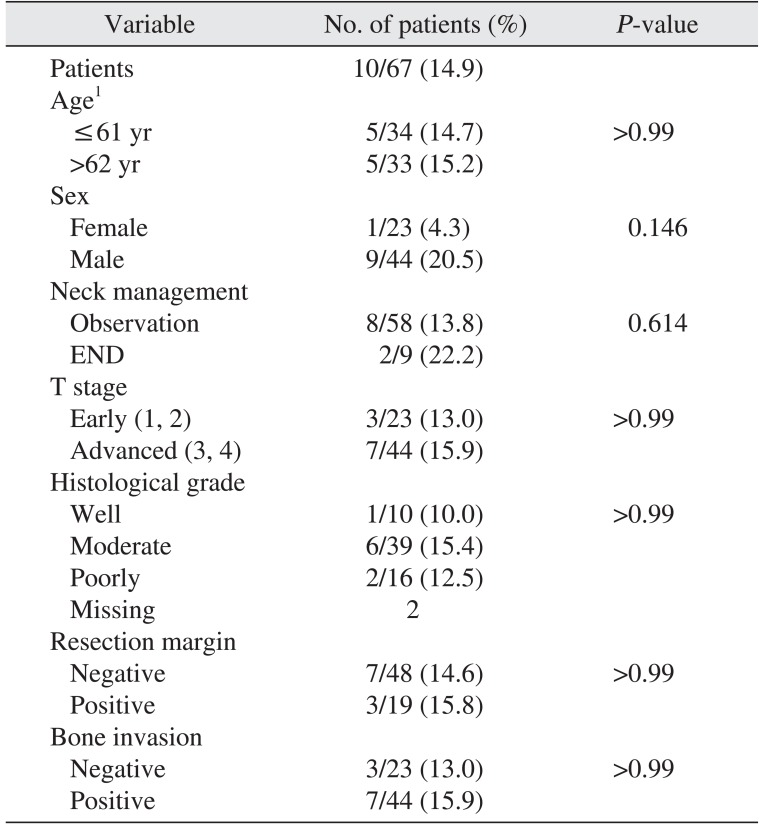
Several factors such as age, sex, site of primary lesion, modality of neck management, T stage, histological grade, resection margin, and bone invasion were analyzed to identify factors that predicted high risk for occult cervical metastasis. (Table 3) No factors were identified as statistically significant predictors of occult cervical metastasis. There was no relationship between advanced T stage and occult cervical metastasis. Neither high histological grade nor bone invasion were associated with incidence of occult cervical metastasis.
Among the 7 patients who had regional recurrence, 5 patients (71.4%) were treated successfully.(Table 2) However, among the 12 patients with local recurrence, treatment for recurrence was successful for only 4 patients (33.3%). Two patients with locoregional recurrence died of uncontrolled disease, so the success rate of recurrence treatment was 0%. Unsurprisingly, all the patients with distant metastases died from cancer. Five-year overall survival rate was 74.0% for the non-END group and 51.9% for the END group. The non-END group survival rate was higher than the END group, but the difference was not statistically significant (P=0.241).(Fig. 1)
Considering morbidity and benefits of neck dissection, END is recommended for patients whose risk of occult cervical metastasis is greater than 15% to 20%110. In the majority of recent studies, the risk of occult cervical metastasis of SCC in the maxillary gingiva was reported to be greater than 20%561112. On the other hand, cervical lymph node metastasis of SCC in the maxillary sinus is reported to be less than 15%7131415. The maxillary sinus is usually considered distinct from the maxillary gingiva when it comes to cancer, but their proximity frequently obscures tumor origin, especially in advanced stages. In this study, the authors defined the primary site of maxillary SCC as maxillary gingiva or maxillary sinus considering anatomical proximity and lymphatic drainages. Risk of occult cervical metastasis of maxillary SCC in the present study was 14.9%. Consistent with other studies, the incidence of maxillary gingiva was higher than that of maxillary sinus (17.1% vs 12.5%, respectively), although slightly less so than in other studies. This incidence is not high enough to recommend END in cN0 patients.
Many studies reported a correlation between advanced maxillary SCC T stage and lymph node metastasis5681617181920. In accordance with their results, Ogura et al.21 reported that radiographical evidence of bone invasion was an indicator of cervical metastasis in SCC of the maxillary gingiva. However, another study of maxillary sinus SCC demonstrated that T1 and T2 stages showed significantly more cervical metastases than T3 and T4 stages and that involvement of other structures was no predictor of cervical metastasis22. Another study also reported T stage was not a significant factor, but that tumor extension to the nasopharynx or oral cavity was a significant factor for neck node metastasis13. In the present study, T stage, histological grade, and bone invasion of the primary tumor were not significant predisposing factors for occult cervical metastasis.
Most studies have focused on the incidence of neck metastasis to prove the necessity for END. The authors believe that the impact of END on patient survival rate should also be considered. A search yielded no study which demonstrated an impact of END on survival rate in SCC of the maxillary sinus. In SCC of the maxillary gingiva, two studies reported a higher 5-year overall survival rate for the END group than for the non-END group, but the differences were not statistically significant56. They also noted that END seemed to confer a survival benefit in advanced T stages. In contrast with their results, the 5-year overall survival rate in this analysis was higher for the non-END than the END group. Small sample size and selection bias (i.e., predilection of END for advanced T stages) may partly account for the results, but the most important factor was the high success rate of treatment for regional recurrence in the non-END group. Many studies reported a low success rate of treatment for regional recurrence, which was one of the reasons for recommending END51617182324. In this study, however, the success rate of treatment for regional recurrence was high (71.4%), in contrast with low success rates of local or locoregional recurrence (33.3% and 0%, respectively). Hence, local control of the primary tumor is more important than modality of neck management for survival of cN0 patients. With complete local control, observation of cN0 neck is recommended when early detection of regional recurrence is possible. Feng et al.6 also reported that early detection could lead to a 100% success rate of cervical salvage irrespective of T stage. The authors believe the key factor in early detection of regional recurrence is patient education with periodic follow-up. In our hospital, in addition to periodic follow-up, patients are instructed about signs of local recurrence such as unhealed ulceration over 2 weeks, swelling, or easy bleeding at the site, and signs of regional recurrence such as a palpable mass in the neck. They are encouraged to come to the hospital as soon as possible to be examined and screened for recurrence if they feel any abnormalities.
This study has the limitations of small sample size, observation period range and surgeon bias for selection of END, such as predilection of END for advanced T stages. A properly-designed study with a larger sample size is needed to confirm the therapeutic value of END in survival of cN0 patients.
The incidence of occult cervical metastasis of maxillary SCC is not high enough to recommend END. For survival of cN0 patients, local control of the primary tumor is more important than modality of neck management. When early detection of regional recurrence is possible, observation of cN0 neck is the treatment of choice irrespective of the site or T stage of maxillary SCC. Because the key enabler of early detection is patient education with periodic follow-up, the patients should be instructed about signs of recurrence and encouraged to come to the hospital as soon as possible if they feel any abnormalities.
References
1. Pitman KT. Rationale for elective neck dissection. Am J Otolaryngol. 2000; 21:31–37. PMID: 10668674.

2. Capote A, Escorial V, Muñoz-Guerra MF, Rodríguez-Campo FJ, Gamallo C, Naval L. Elective neck dissection in early-stage oral squamous cell carcinoma--does it influence recurrence and survival? Head Neck. 2007; 29:3–11. PMID: 17103411.

3. Balasundram S, Mustafa WM, Ip J, Adnan TH, Supramaniam P. Conservative neck dissection in oral cancer patients: a 5 year retrospective study in Malaysia. Asian Pac J Cancer Prev. 2012; 13:4045–4050. PMID: 23098514.

4. Krishna Rao SV, Mejia G, Roberts-Thomson K, Logan R. Epidemiology of oral cancer in Asia in the past decade--an update (2000-2012). Asian Pac J Cancer Prev. 2013; 14:5567–5577. PMID: 24289546.
5. Poeschl PW, Seemann R, Czembirek C, Russmueller G, Sulzbacher I, Selzer E, et al. Impact of elective neck dissection on regional recurrence and survival in cN0 staged oral maxillary squamous cell carcinoma. Oral Oncol. 2012; 48:173–178. PMID: 21974917.

6. Feng Z, Li JN, Li CZ, Guo CB. Elective neck dissection versus observation for cN0 neck of squamous cell carcinoma primarily located in the maxillary gingiva and alveolar ridge: a retrospective study of 129 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116:556–561. PMID: 24119520.

7. Brown JS, Bekiroglu F, Shaw RJ, Woolgar JA, Triantafyllou A, Rogers SN. First report of elective selective neck dissection in the management of squamous cell carcinoma of the maxillary sinus. Br J Oral Maxillofac Surg. 2013; 51:103–107. PMID: 22578881.

8. Abu-Ghanem S, Horowitz G, Abergel A, Yehuda M, Gutfeld O, Carmel NN, et al. Elective neck irradiation versus observation in squamous cell carcinoma of the maxillary sinus with N0 neck: a meta-analysis and review of the literature. Head Neck. 2015; 37:1823–1828. PMID: 24913744.

9. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010; 17:1471–1474. PMID: 20180029.

10. Weiss MH, Harrison LB, Isaacs RS. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch Otolaryngol Head Neck Surg. 1994; 120:699–702. PMID: 8018319.
11. Montes DM, Schmidt BL. Oral maxillary squamous cell carcinoma: management of the clinically negative neck. J Oral Maxillofac Surg. 2008; 66:762–766. PMID: 18355602.

12. Simental AA Jr, Johnson JT, Myers EN. Cervical metastasis from squamous cell carcinoma of the maxillary alveolus and hard palate. Laryngoscope. 2006; 116:1682–1684. PMID: 16955004.

13. Kim GE, Chung EJ, Lim JJ, Keum KC, Lee SW, Cho JH, et al. Clinical significance of neck node metastasis in squamous cell carcinoma of the maxillary antrum. Am J Otolaryngol. 1999; 20:383–390. PMID: 10609483.
14. Giri SP, Reddy EK, Gemer LS, Krishnan L, Smalley SR, Evans RG. Management of advanced squamous cell carcinomas of the maxillary sinus. Cancer. 1992; 69:657–661. PMID: 1730116.

15. Valentini V, Terenzi V, Battisti A, Cassoni A, Anelli A, Priore P, et al. Management of clinically negative neck in maxillary carcinoma. J Craniofac Surg. 2010; 21:759–762. PMID: 20485042.

16. Le QT, Fu KK, Kaplan MJ, Terris DJ, Fee WE, Goffinet DR. Lymph node metastasis in maxillary sinus carcinoma. Int J Radiat Oncol Biol Phys. 2000; 46:541–549. PMID: 10701732.

17. Montes DM, Carlson ER, Fernandes R, Ghali GE, Lubek J, Ord R, et al. Oral maxillary squamous carcinoma: an indication for neck dissection in the clinically negative neck. Head Neck. 2011; 33:1581–1585. PMID: 21990223.

18. Morris LG, Patel SG, Shah JP, Ganly I. High rates of regional failure in squamous cell carcinoma of the hard palate and maxillary alveolus. Head Neck. 2011; 33:824–830. PMID: 20949448.

19. Lin HW, Bhattacharyya N. Survival impact of nodal disease in hard palate and maxillary alveolus cancer. Laryngoscope. 2009; 119:312–315. PMID: 19172612.

20. Beltramini GA, Massarelli O, Demarchi M, Copelli C, Cassoni A, Valentini V, et al. Is neck dissection needed in squamous-cell carcinoma of the maxillary gingiva, alveolus, and hard palate? A multicentre Italian study of 65 cases and literature review. Oral Oncol. 2012; 48:97–101. PMID: 21993155.

21. Ogura I, Kurabayashi T, Sasaki T, Amagasa T, Okada N, Kaneda T. Maxillary bone invasion by gingival carcinoma as an indicator of cervical metastasis. Dentomaxillofac Radiol. 2003; 32:291–294. PMID: 14709602.

22. Paulino AC, Fisher SG, Marks JE. Is prophylactic neck irradiation indicated in patients with squamous cell carcinoma of the maxillary sinus? Int J Radiat Oncol Biol Phys. 1997; 39:283–289. PMID: 9308929.

23. Yagi K, Fukuda S, Furuta Y, Oridate N, Homma A, Nagahashi T, et al. A clinical study on the cervical lymph node metastasis of maxillary sinus carcinoma. Auris Nasus Larynx. 2001; 28:S77–S81. PMID: 11683349.

24. Mourouzis C, Pratt C, Brennan PA. Squamous cell carcinoma of the maxillary gingiva, alveolus, and hard palate: is there a need for elective neck dissection? Br J Oral Maxillofac Surg. 2010; 48:345–348. PMID: 19665264.

Fig. 1
Kaplan-Meier survival curves of overall survival for non-END group versus END group. (END: elective neck dissection)

Table 1
Study population characteristics
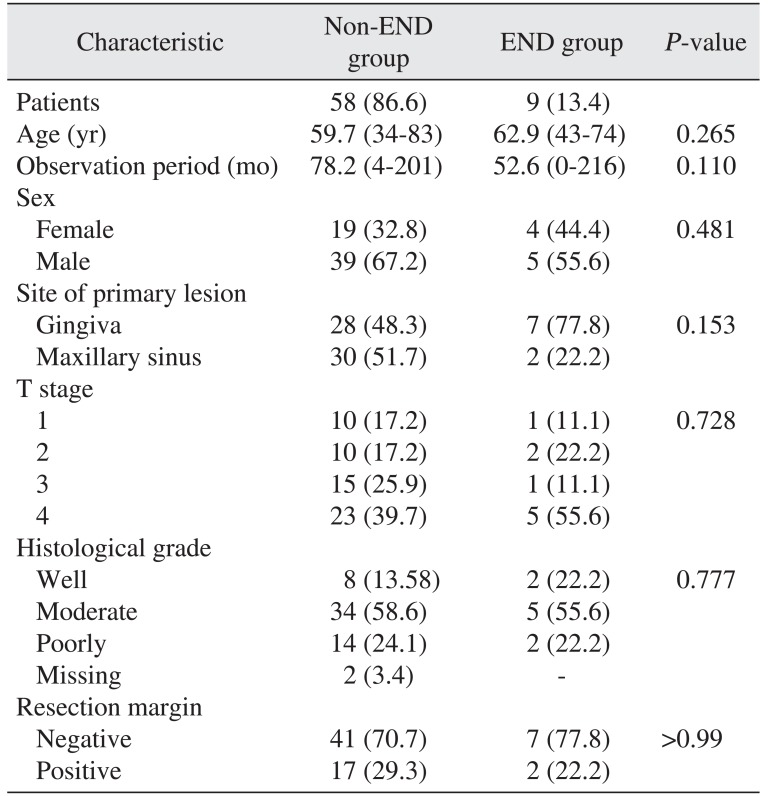
Table 2
The patterns of recurrence and results of recurrence treatment
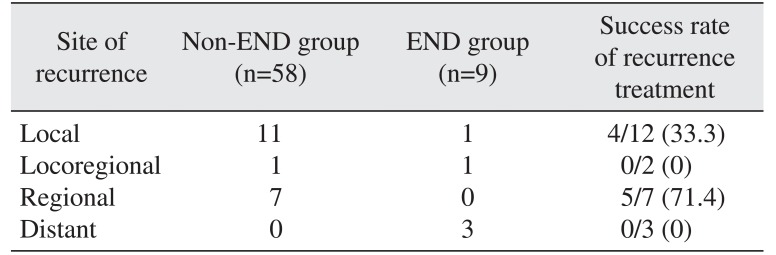
| Site of recurrence | Non-END group (n=58) | END group (n=9) | Success rate of recurrence treatment |
|---|---|---|---|
| Local | 11 | 1 | 4/12 (33.3) |
| Locoregional | 1 | 1 | 0/2 (0) |
| Regional | 7 | 0 | 5/7 (71.4) |
| Distant | 0 | 3 | 0/3 (0) |
Table 3
Analysis of the predisposing factors for occult metastasis




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download