Abstract
Objectives
The purpose of this study was to evaluate and compare the treatment outcomes of partial glossectomy with or without elective neck dissection in patients with tongue squamous cell carcinoma (SCCa).
Materials and Methods
A total of 98 patients who were diagnosed with tongue SCCa and underwent partial glossectomy between 2005 and 2014 were evaluated. Only 14 patients received elective neck dissection, and 84 patients received only partial glossectomy.
Results
There were 56 men and 42 women with a mean age of 57 years and mean follow-up period of 33.7 months. There were 70 patients graded as T1 and 28 as T2. The total occult metastasis rate was 17.3%. The 5-year overall survival rate was 83.3% with elective neck dissection and 92.4% with observation. The 5-year disease-free survival rate was in 70.7% in the elective neck dissection group and 65.3% in the observation group.
Conclusion
We retrospectively reviewed the records of 98 patients with tongue SCCa. These patients were divided into two groups, those who underwent elective neck dissection and those who did not. There was no statistically significant difference between the groups undergoing partial glossectomy with or without elective neck dissection.
Oral cancer is the sixth most common cancer, and its incidence is currently increasing worldwide12. Squamous cell carcinoma (SCCa) of mouth area accounts for more than 90% of all malignant neoplasms in the oral area34. Tongue is the most common site of primary SCCa of the mouth area5.
The most important prognosticator of survival in tongue SCCa is the presence of lymph node metastasis678. In tongue SCCa, the incidence of occult neck metastasis is relatively high; 7%-28% of patients with T1 and 28%-40% of T2 SCCa of the tongue were clinically staged as N0191011. Another study showed the incidence of occult neck metastasis in T1 as 13%-33% and 37%-53% in T212131415. Occult metastasis in T1 is much less frequent than that in T2. The effectiveness of elective neck dissection in patients with clinically negative lymph node (N0) in stage I or II (early) SCCa of the tongue remains controversial112151617181920.
We retrospectively compared the results of elective neck dissection with observation in the treatment of early clinically negative neck in tongue SCCa. We also attempted to evaluate whether elective neck dissection can increase survival time and estimate the value of elective neck dissection in clinically stage I or II tongue SCCa.
Patients with stage I or stage II SCCa of the tongue who were treated surgically in the Department of Oral and Maxillofacial Surgery, Seoul National University Dental Hospital (Seoul, Korea) between January 2005 and May 2014 were reviewed. All patients had preoperative clinically N0 status and had undergone partial glossectomy with or without elective neck dissection as part of the primary surgical treatment. Patients who had non-SCCa and received radiotherapy or chemotherapy after surgical treatment were excluded. Staging was evaluated according to the 2010 American Joint Committee on Cancer (AJCC) criteria. All patients were staged N0 after clinical and laboratory assessment and preoperative diagnostic examination including palpation and imaging of the neck such as computed tomography, ultrasonography, and magnetic resonance imaging. This study was reviewed by the Institutional Review Board of Seoul National University School of Dentistry (IRB No. S-D20160012).
The patients were divided into two groups according to management of the neck node. The patients in the partial glossectomy without elective neck dissection group had not received any kind of neck treatment. The elective neck dissection group consisted of patients who had undergone prophylactic neck dissection.
The two groups were compared with respect to overall survival (OS) and disease-free survival (DFS). OS was time between the date of first visit to the date of last follow-up or death from any cause. DFS was time from the date of first visit to the date of recurrence. Patients lost to follow-up were included in all analyses, until the date of last follow-up.
All data for statistical analysis were compiled using the IBM SPSS Statistics for Windows (version 21.0; IBM Co., Armonk, NY, USA). Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Cox regression analysis was performed for multi-variance analysis. Statistical significance was indicated by P<0.05.
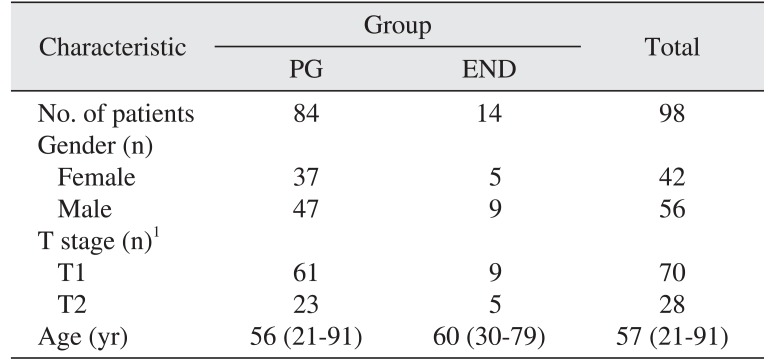
The 98 patients comprised 56 male and 42 female. Patient age ranged from 21 to 91 years with a mean of 57 years, and the mean postoperative follow-up period was 33.7 months. The clinical TNM stage was T1N0M0 in 70 patients and T2N0M0 in 28 patients. All patients were staged N0 by clinical and radiologic assessment.
The most common primary location was the lateral tongue border (70 patients), followed by ventral side (20 patients), dorsal side (6 patients), and tip of tongue (2 patients). Left side location was noted in 65 patients and right side in 33 patients. Histopathologically, there were 66 well differentiated, 6 moderately differentiated, and 11 less well differentiated tumors. Differentiation was not documented in 15 patients.
All 98 patients received partial glossectomy, with 14 patients receiving partial glossectomy with elective neck dissection and 84 patients receiving partial glossectomy only. The partial glossectomy only group (PG group) consisted of 84 patients (47 males, 37 females). Of these, 61 patients had a T1 primary tumor, while 23 patients had a T2 primary tumor. The partial glossectomy with elective neck dissection group (END group) consisted of 14 patients (9 males, 5 females), 9 of whom had T1 primary tumor, while 5 patients had T2 primary tumor. The patients are described in Table 1.
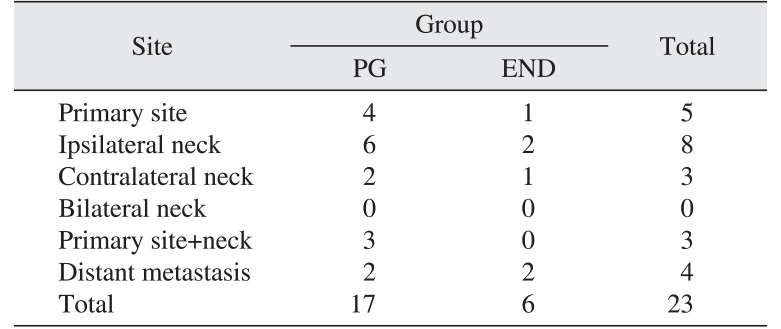
Of the total cases, 17 experienced occult cervical metastasis, including pathologically positive metastasis in 4 cases in the elective neck dissection group and neck metastasis in 13 cases in the primary resection without elective neck dissection group. The total prevalence of occult metastasis was 17.3%. The incidence of T1 and T2 occult metastasis were 17.1% and 17.9%, respectively.
In the PG group, a total of 15 patients experienced locoregional recurrence, located as follows: primary site only (4 cases), primary site+neck (3 cases), and neck only (8 cases). All patients had surgical salvage. Distant metastasis occurred in 2 patients, both of whom had also experienced previous locoregional recurrence. In the END group, a total of 4 patients demonstrated locoregional recurrence, located as follows: primary site only (1 case) and neck only (3 cases). All patients had surgical salvage. Distant metastasis occurred in 2 patients, and 1 patient had previously been diagnosed with locoregional recurrence.(Table 2)
The 5-year OS rate for all patients was 90.8%, and the DFS rate was 67.1%. The 5-year DFS rates of T1 and T2 were 71.1% and 54.3%, respectively.
The 5-year OS and 5-year DFS rates for the PG group were 92.4% and 65.3%, respectively. The 5-year OS and 5-year DFS rates for the END group were 83.3% and 70.7%, respectively.(Table 3) By log-rank test, the OS (P=0.325) and DFS (P=0.728) were not significantly different between the PG and END groups.(Fig. 1, 2)
The 5-year OS and DFS rates of the PG and END groups in T1 and T2 patients are shown in Tables 4 and 5, respectively. By log-rank test, the DFS was not significant statistically different between the PG and END groups in T1 (P=0.961) or T2 (P=0.765).(Fig. 3, 4)
Univariate analysis demonstrated that patients with recurrence had significantly shorter OS and DFS. Age, gender, tumor site, histological grade, elective neck dissection, and T staging were not prognostic factors. By multivariate analysis, recurrence and gender were independent factors affecting DFS. Elective neck dissection showed no prognostic effect in OS, and recurrence was the only independent factor affecting OS.(Table 6)
We retrospectively analyzed our results of the efficacy of elective neck dissection in stage I and II tongue SCCa with clinically N0 grading in the neck.
The presence of cervical lymph node metastasis is a major determinant of prognosis and treatment in tongue SCCa. As mentioned before, the occult metastasis rate ranged from 7%-33% in T1 and 28%-53% in T2 in the clinically N0 tongue SCCa patients. In this study, the risk of occult neck metastasis also increased according to T stage. The overall rate of occult metastasis was 17.3% (17.1% in T1 and 17.8% in T2), which was comparable with the occult metastasis in other studies.
Our data demonstrated that the 5-year OS rate in the PG and END groups was 92.4% and 83.3%, respectively, and the 5-year DFS rate in the PG and END groups was 65.3% and 70.7%. There was no significant difference between the two groups. The results from our study show that END cannot significantly improve survival for patients with stage I or II tongue SCCa compared with PG. Other studies also found it difficult to show an improvement of survival after elective neck dissection. Keski-Säntti et al.15 reviewed 80 patients with T1 or T2 with clinically N0 neck nodes in tongue SCCa and reported that elective neck dissection did not increase DFS or OS. Liu et al.1 demonstrated no statistical differences in DFS or OS between END and PG groups. In contrast, Kligerman et al.21 reported that elective neck dissection increased the 3-year survival rate from 49% to 72%.
In T1 patients, the 5-year DFS rate for the PG and END groups was 68.8% and 77.8%, respectively. In T2 patients, this 5-year DFS rate was 51.0% and 60.0%, respectively.
As shown in this study, gender and recurrence were independent factors affecting DFS, while only recurrence was an independent factor affecting OS. Elective neck dissection was not an independent predictive factor of OS or DFS by univariate or multivariate analysis. Therefore, elective neck dissection could not significantly improve the OS or DFS in early (stage I or II) tongue SCCa. Age, histological grade, tumor site, and stage also showed no prognostic effect in stage I or stage II tongue SCCa.
In conclusion, this study showed that elective neck dissection did not have a significant benefit in terms of survival in the management of clinically N0 neck of early stage tongue SCCa. Routine recommendation of elective neck dissection of clinically N0 neck is not advisable. We conclude that careful selection of elective neck dissection can be of help to the patient. In the future, a prospective randomized study will be useful to further evaluate the benefit of neck management in the treatment of the early stage (stage I or II) tongue SCCa.
References
1. Liu TR, Chen FJ, Yang AK, Zhang GP, Song M, Liu WW, et al. Elective neck dissection in clinical stage I squamous cell carcinoma of the tongue: does it improve regional control or survival time? Oral Oncol. 2011; 47:136–141. PMID: 21216182.

2. Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999; 49:8–31. 1PMID: 10200775.

3. Bagan J, Sarrion G, Jimenez Y. Oral cancer: clinical features. Oral Oncol. 2010; 46:414–417. PMID: 20400366.

4. Ow TJ, Myers JN. Current management of advanced resectable oral cavity squamous cell carcinoma. Clin Exp Otorhinolaryngol. 2011; 4:1–10. PMID: 21461056.

5. Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck. 2002; 24:165–180. PMID: 11891947.

6. Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990; 160:405–409. PMID: 2221244.

7. Koo BS, Lim YC, Lee JS, Choi EC. Recurrence and salvage treatment of squamous cell carcinoma of the oral cavity. Oral Oncol. 2006; 42:789–794. PMID: 16455287.

8. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008; 371:1695–1709. PMID: 18486742.

9. Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004; 131:472–476. PMID: 15467620.

10. Yuen AP, Lam KY, Chan AC, Wei WI, Lam LK, Ho WK, et al. Clinicopathological analysis of elective neck dissection for N0 neck of early oral tongue carcinoma. Am J Surg. 1999; 177:90–92. PMID: 10037317.
11. Akhtar S, Ikram M, Ghaffar S. Neck involvement in early carcinoma of tongue. Is elective neck dissection warranted? J Pak Med Assoc. 2007; 57:305–307. PMID: 17629233.
12. Haddadin KJ, Soutar DS, Oliver RJ, Webster MH, Robertson AG, MacDonald DG. Improved survival for patients with clinically T1/T2, N0 tongue tumors undergoing a prophylactic neck dissection. Head Neck. 1999; 21:517–525. PMID: 10449667.

13. Teichgraeber JF, Clairmont AA. The incidence of occult metastases for cancer of the oral tongue and floor of the mouth: treatment rationale. Head Neck Surg. 1984; 7:15–21. PMID: 6490380.

14. Po Wing, Lam KY, Lam LK, Ho CM, Wong A, Chow TL, et al. Prognostic factors of clinically stage I and II oral tongue carcinoma: a comparative study of stage, thickness, shape, growth pattern, invasive front malignancy grading, Martinez-Gimeno score, and pathologic features. Head Neck. 2002; 24:513–520. PMID: 12112547.
15. Keski-Säntti H, Atula T, Törnwall J, Koivunen P, Mäkitie A. Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral tongue. Oral Oncol. 2006; 42:96–101. PMID: 16256414.

16. Jalisi S. Management of the clinically negative neck in early squamous cell carcinoma of the oral cavity. Otolaryngol Clin North Am. 2005; 38:37–46. viiiPMID: 15649497.

17. Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009; 31:765–772. PMID: 19408291.

18. Lim YC, Lee JS, Koo BS, Kim SH, Kim YH, Choi EC. Treatment of contralateral N0 neck in early squamous cell carcinoma of the oral tongue: elective neck dissection versus observation. Laryngoscope. 2006; 116:461–465. PMID: 16540910.

19. Werning JW, Heard D, Pagano C, Khuder S. Elective management of the clinically negative neck by otolaryngologists in patients with oral tongue cancer. Arch Otolaryngol Head Neck Surg. 2003; 129:83–88. PMID: 12525200.

20. Persky MS, Lagmay VM. Treatment of the clinically negative neck in oral squamous cell carcinoma. Laryngoscope. 1999; 109:1160–1164. PMID: 10401861.

21. Kligerman J, Lima RA, Soares JR, Prado L, Dias FL, Freitas EQ, et al. Supraomohyoid neck dissection in the treatment of T1/T2 squamous cell carcinoma of oral cavity. Am J Surg. 1994; 168:391–394. PMID: 7977957.

Fig. 1
Overall survival rates of PG group and END group. (PG: patients had partial glossectomy only, END: patients who had elective neck treatment)
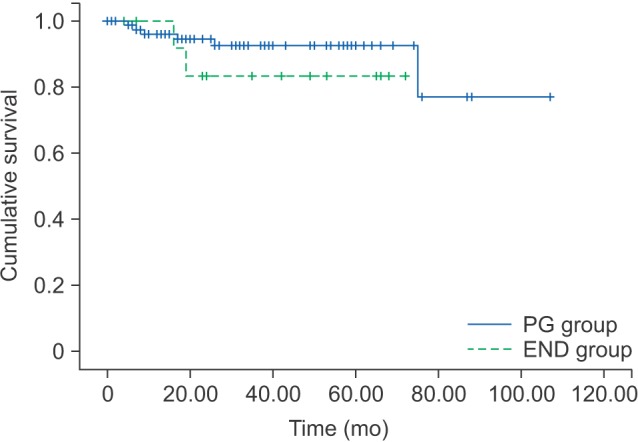
Fig. 2
Disease-free survival rates of PG group and END group. (PG: patients had partial glossectomy only, END: patients who had elective neck treatment)
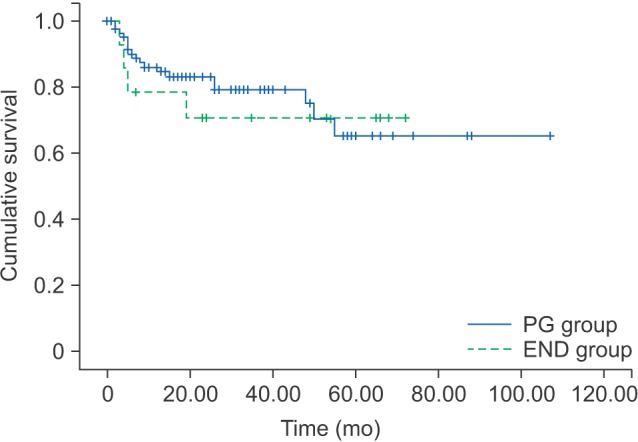
Fig. 3
Disease-free survival rates of PG group and END group in T1 patients. (PG: patients had partial glossectomy only, END: patients who had elective neck treatment)
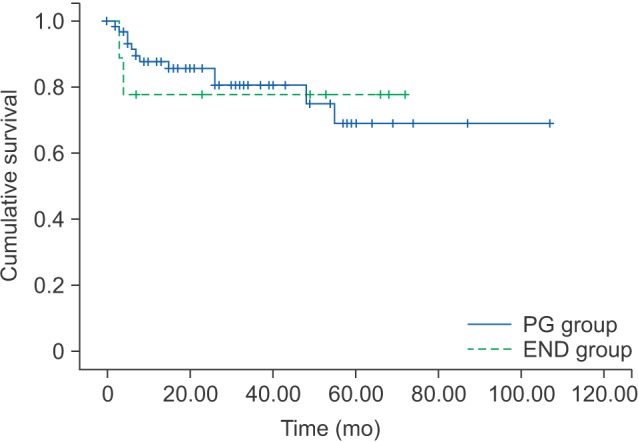
Fig. 4
Disease-free survival rates of PG group and END group in T2 patients. (PG: patients had partial glossectomy only, END: patients who had elective neck treatment)
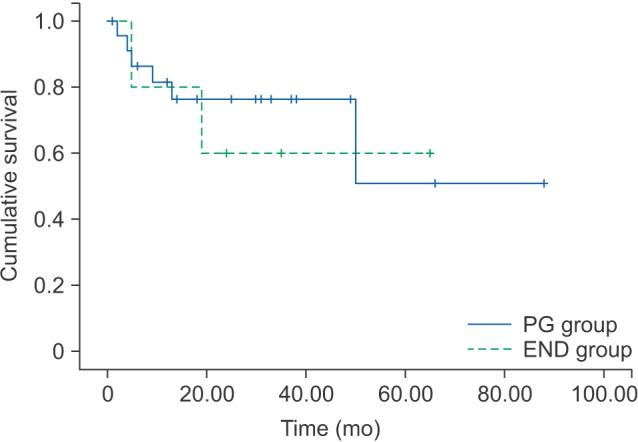
Table 1
Characteristics of patients
| Characteristic | Group | Total | |
|---|---|---|---|
| PG | END | ||
| No. of patients | 84 | 14 | 98 |
| Gender (n) | |||
| Female | 37 | 5 | 42 |
| Male | 47 | 9 | 56 |
| T stage (n)1 | |||
| T1 | 61 | 9 | 70 |
| T2 | 23 | 5 | 28 |
| Age (yr) | 56 (21–91) | 60 (30–79) | 57 (21–91) |
Table 2
Locoregional recurrences and distant metastasis
| Site | Group | Total | |
|---|---|---|---|
| PG | END | ||
| Primary site | 4 | 1 | 5 |
| Ipsilateral neck | 6 | 2 | 8 |
| Contralateral neck | 2 | 1 | 3 |
| Bilateral neck | 0 | 0 | 0 |
| Primary site+neck | 3 | 0 | 3 |
| Distant metastasis | 2 | 2 | 4 |
| Total | 17 | 6 | 23 |
Table 3
Survival rate in the two groups

| Group | No. of patients | 3-year OS (%) | 5-year OS (%) | 3-year DFS (%) | 5-year DFS (%) |
|---|---|---|---|---|---|
| PG | 84 | 92.4 | 92.4 | 79.1 | 65.3 |
| END | 14 | 83.3 | 83.3 | 70.7 | 70.7 |
| Total | 98 | 90.8 | 90.8 | 77.8 | 67.1 |
Table 4
Survival rate of T1 patients in the two groups

| Group | No. of patients | 3-year OS (%) | 5-year OS (%) | 3-year DFS (%) | 5-year DFS (%) |
|---|---|---|---|---|---|
| PG | 61 | 93.5 | 93.5 | 80.5 | 68.8 |
| END | 9 | 0100 | 0100 | 77.8 | 77.8 |
| Total | 70 | 94.3 | 94.3 | 80.1 | 71.1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download