Abstract
Non-Hodgkin's lymphoma on the parotid gland is a relatively rare occurrence among head and neck tumors. The mass of parotid gland lymphoma cannot be distinguished from other benign masses of the parotid gland; therefore, it is important to consider lymphoma in the differential diagnosis when examining parotid swellings and masses. Parotid gland lymphoma is most likely to be B-cell, non-Hodgkin's lymphoma of one of three types, which include follicular, marginal zone, and diffuse large B-cell, although other histologic patterns have been described. We present a review of a patient with diffuse large B-cell lymphoma (DLBCL) who presented to the Department of Oral and Maxillofacial Surgery of Pusan National University Hospital (Yangsan, Korea).
Malignant lymphoma is a group of diseases which have a wide variety of clinical, histological features, genetic abnormalities and immunophenotypes12. Malignant lymphomas can be categorized into two major subtypes, Hodgkin's lymphoma (HL) and non-Hodgkin's lymphoma (NHL). Lymphomas derived from T-cells, B-cells and NK cells belong to a group of NHL3. HL usually appears as a node-type disease including inguinal, axillary and cervical nodes. Whereas, NHL localizes extra-nodally in the digestive tract, salivary glands and rarely the jaw4. The latter group has the most prevalence of all lymphomas in the head and neck, accounting for 75% of cases5. Diffuse large B-cell lymphoma (DLBCL) is the most common NHL type in the head and neck area6.
Approximately a quarter of all lymphomas on the extra nodes develop in the head and neck, principally in the parotid glands, tonsils and pharynx7. Among tumors of the parotid, the prevalence of lymphoma is rare, representing 1% to 4% of cases8. When a clinician evaluates a new parotid mass, lymphoma is often not considered9. Clinical or radiographic features providing a definitive diagnosis are not distinguishable. Because of these difficulties, surgical procedures are undertaken, such as parotidectomy10.
Regarding therapy, localized low-grade lymphomas can be treated with radiotherapy only, whereas diffuse high-grade types are treated with aggressive chemotherapy. A combination of radiotherapy and chemotherapy is used to treat patients with localized high-grade lymphomas11. Although DLBCL is an aggressive, rapidly growing neoplasm, in this case the lesion was localized. It seemed that a combination of chemotherapy and radiotherapy might be the appropriate choice. Nevertheless, a surgery in the case of extra nodal DLBCL involvement was necessary to obtain a specimen sufficient for a complete histological examination and treatment planning. We report a female patient underwent surgery for parotid lymphoma (DLBCL).
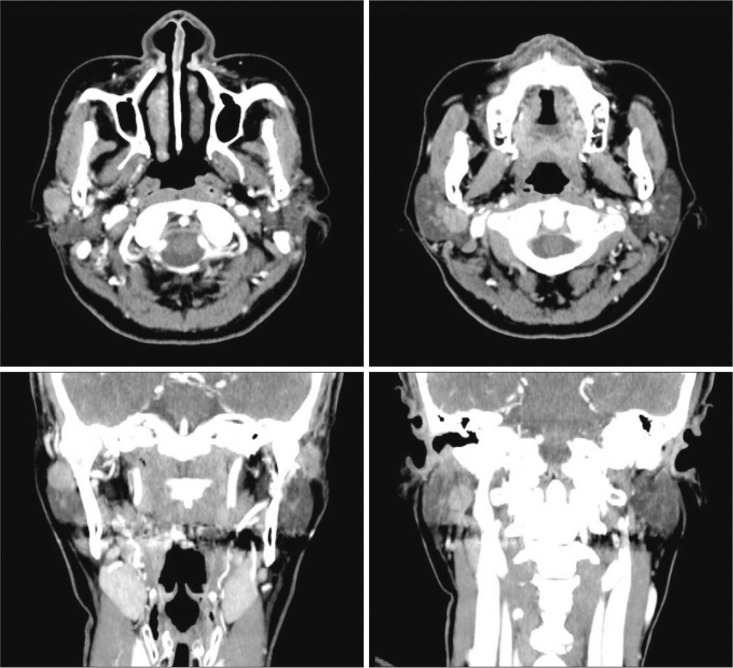
In June 2014, a 54-year-old female visited the Department of Otolaryngology, Pusan National University Hospital (Yangsan, Korea), complaining of a painless lesion on her right cheek that appeared 10 days before her visit. Oral examination showed salivation function on Stensen's duct was within the normal range, and peri-ductal swelling was observed. She had punch biopsy treatment on her oral mucosa by a doctor of otolaryngology. The biopsy result showed benign squamous epithelium. A contrast-enhanced computed tomography (CT) was performed on her head and neck.(Fig. 1) After a radiologic diagnosis for her CT was established as Warthin's tumor, the patient was referred to the Department of Oral and Maxillofacial Surgery for surgery.
Subtotal parotidectomy was performed for lesion removal and to make a definite diagnosis. There was some adhesion between the lesion and the facial nerve, and the lesion was scattered on and beneath the nerve.(Fig. 2. A, 2. B) The fibrous tissue around the Stensen's duct and lymph node were removed, which was enlarged and under the parotid gland. (Fig. 2. C) Frozen biopsy during the operation showed malignant and lymphoid tissue.
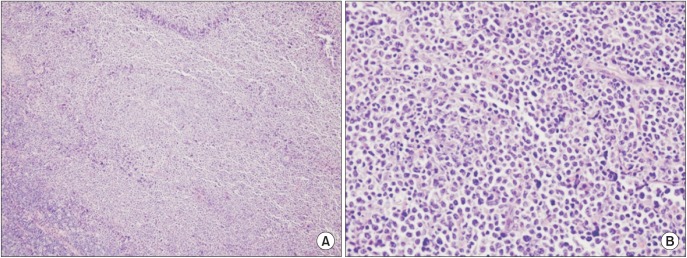
The biopsy specimen revealed a malignant proliferation of undifferentiated large cells with abundant cytoplasm under a microscope. Hodgkin cells and Reed-Sternber cells were not observed in any regions.(Fig. 3) Immunohistochemistry was positive for bcl6, CD20, and MUM1.(Fig. 4) but was negative for S100, HMB 45, and vimentin. All together, these findings suggested a monoclonal malignancy composed of lymphoid cells of B-cell origin.
The patient was treated with positron emission tomography-computed tomography (PET-CT) (18-fluorodeoxyglucose [FDG]) to identify metastases. FDG uptake increased in the left breast at the upper outer quadrant.(Fig. 5) Needle biopsy was conducted and showed atypical lymphatic infiltration combined with DLBCL. The patient was referred to an oncologist for chemotherapy. She received chemotherapy 6 times for 5 months after surgery using R-CHOP with Neulasta (pegfilgrastim; Amgen, Thousand Oaks, CA, USA). Contrast-enhanced CT of the face was taken at check-up 3 times until January 2016. No changes were seen on follow-up CTs except normal postoperative healing. Any evidence of recurrence has not been seen in clinical or radiological examinations until March 2016. Facial nerve weakness, which was observed for a month after surgery, had resolved.
Malignant lymphomas constitute a neoplastic proliferation group arising from lymphocytes. Multi-nucleated Reed-Sternberg cells histologically characterize Hodgkin's disease. All other lymphoid neoplasms are classified as NHL and originate mostly from B-lymphocytes12. Only 1%-4% of all parotid tumors are finally diagnosed as primary parotid NHL8.
Not all lymphomas associated with salivary glands are extra nodal in origin. Plentiful lymph nodes surround the parotid gland and into the gland. Nodal lymphomas may replace these lymph nodes that can then replace the parotid parenchyma secondarily. On histological evaluation, it may be difficult to decide the origin site if molecular and immunophenotypic analysis are not undertaken13. The patient in this report had an enlarged lymph node under the parotid mass (Fig. 2. B), but we cannot know the site of the DLBCL origin.
Clinical examination procedures can't be used to distinguish between a malignant or benign parotid mass. Malignant lymphoma should be considered as the final diagnosis14. If a patient presenting with a parotid mass has Sjögren syndrome (underlying autoimmune disorder), the morbidity rate of lymphoma is reported to be as high as 44%15.
Initial evaluation for parotid tumors should include magnetic resonance imaging (MRI) or CT to determine tumor size, shape, and location16. MRI and CT have been considered to be equally effective when evaluating tumor bounds, location, and size. But, the benefits of CT compared with MRI include lower cost, increased accessibility and more rapid results17. PET scanning (18-FDG) is also often obtained when treating NHL for considering further treatment plans, to decide exact prognosis, and also to serve as a way to detect a recurrence or lymph node reactions within the postoperation course. PET scanning is reported to have 90% specificity and 80% sensitivity for identifying malignancy18. PET-CT in this report played a role in discovering a suspicious mass in the patient's breast.
Radiotherapy and chemotherapy are common treatments for NHL. Aggressive lymphomas like DLBCL are assumed to have disseminated lesions, even if there is no clear evidence on radiologic exams, so they are treated with chemotherapy and rituximab-CHOP. Rituximab-CHOP is generally provided every 3 weeks for 6-8 cycles. In this case, 6 cycles were prescribed at proper intervals while monitoring the patient for renal and hepatic damage, neurotoxicity, neutropenia and thrombocytopenia19. Regarding therapy, localized low-grade lymphoma is treated with radiography, whereas massive chemotherapy is provided for diffuse high-grade cases. A combination of radiotherapy and chemotherapy is used to treat patients with localized high-grade lymphomas. Surgery can complement radiotherapy by providing a specimen sufficient for a complete histological examination7. DLBCL is an aggressive, rapidly growing neoplasm. Fortunately the lesion in this case was localized to the right parotid gland and right breast as seen on radiologic scans. It seemed that a combination of chemotherapy and surgery would be the most appropriate choice. Prognosis is good in low-grade or localized neoplasms, whereas in the case of disseminated neoplasms, it is unfavorable1.
Detection of a parotid mass is a frequent event in dental or medical practices and patients with parotid gland extra nodal lymphoma may not be distinguished clinically from those with a benign lesion like Warthin's tumor or pleomorphic adenoma. Diagnostic steps must include CT or MRI for radiological diagnosis and treatment plan development. A fine needle aspiration biopsy (FNAB) may be helpful in some cases. Clinicians can rule out diverse probable diagnoses with an FNAB, especially combined with immunophenotyping and flow cytometry. FNAB using flow cytometry may be a well-established process aiding final diagnosis and differential diagnosis between lymphoma sub-types20. In spite of FNAB sensitivity and specificity for identifying malignant lymphoma, its sensitivity and specificity with respect to final histologic sub-type variables are less robust16. And targeting error is a common occurrence in FNAB. The targeting error which really happened during punch biopsy (similar to FNAB) performance helped us to determine surgery was required.
Actually, most patients require a superficial or total parotidectomy at final diagnosis, because frozen sectional biopsy and FNAB are often not reliable for making a definitive diagnosis. A thorough assessment and staging decision is necessary before regular treatment. Surgery can identify a mass as a specific subtype or grade of malignant lymphoma16. Patients who have an early stage parotid gland DLBCL have been shown to have a better prognosis10. The final treatment protocol which included surgery and chemotherapy produced a satisfactory result with no recurrence for 19 months. In conclusion, parotidectomy surgery to remove a local parotid gland DLBCL can be a proper treatment option instead of radiotherapy, especially if combined with chemotherapy.
References
1. Huh J. Epidemiologic overview of malignant lymphoma. Korean J Hematol. 2012; 47:92–104. PMID: 22783355.

2. Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117:5019–5032. PMID: 21300984.

3. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Thiele J, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC;2008. p. 9–32.
4. Wolvius EB, van der Valk P, van der Wal JE, van Diest PJ, Huijgens PC, van der Waal I, et al. Primary extranodal non-Hodgkin lymphoma of the oral cavity. An analysis of 34 cases. Eur J Cancer B Oral Oncol. 1994; 30B:121–125. PMID: 8032301.

5. Boring CC, Squires TS, Tong T. Cancer statistics, 1993. CA Cancer J Clin. 1993; 43:7–26. PMID: 8422609.

6. Iguchi H, Wada T, Matsushita N, Oishi M, Yamane H. Anatomic distribution of hematolymphoid malignancies in the head and neck: 7 years of experience with 122 patients in a single institution. Acta Otolaryngol. 2012; 132:1224–1231. PMID: 23025415.

7. Zapater E, Bagán JV, Carbonell F, Basterra J. Malignant lymphoma of the head and neck. Oral Dis. 2010; 16:119–128. PMID: 20374502.

8. Mehle ME, Kraus DH, Wood BG, Tubbs R, Tucker HM, Lavertu P. Lymphoma of the parotid gland. Laryngoscope. 1993; 103:17–21.

9. Loggins JP, Urquhart A. Preoperative distinction of parotid lymphomas. J Am Coll Surg. 2004; 199:58–61. PMID: 15217631.
10. Dispenza F, Cicero G, Mortellaro G, Marchese D, Kulamarva G, Dispenza C. Primary non-Hodgkins lymphoma of the parotid gland. Braz J Otorhinolaryngol. 2011; 77:639–644. PMID: 22030974.

11. Abbaszadeh-Bidokhty H, Mohtasham N, Pazouki M, Babakoohi S. Primary diffuse large B-cell lymphoma of the mandible: a case report. J Oral Maxillofac Surg Med Pathol. 2014; 26:98–100.
12. Eisenbud L, Sciubba J, Mir R, Sachs SA. Oral presentations in non-Hodgkin's lymphoma: a review of thirty-one cases. Part II. Fourteen cases arising in bone. Oral Surg Oral Med Oral Pathol. 1984; 57:272–280. PMID: 6584818.
13. Barnes L, Myers EN, Prokopakis EP. Primary malignant lymphoma of the parotid gland. Arch Otolaryngol Head Neck Surg. 1998; 124:573–577. PMID: 9604985.

14. Shum JW, Emmerling M, Lubek JE, Ord RA. Parotid lymphoma: a review of clinical presentation and management. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 118:e1–e5. PMID: 24405648.

15. Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the Inter-Lymph Consortium. Blood. 2008; 111:4029–4038. PMID: 18263783.

16. Feinstein AJ, Ciarleglio MM, Cong X, Otremba MD, Judson BL. Parotid gland lymphoma: prognostic analysis of 2140 patients. Laryngoscope. 2013; 123:1199–1203. PMID: 23576299.

17. Koyuncu M, Seşen T, Akan H, Ismailoglu AA, Tanyeri Y, Tekat A, et al. Comparison of computed tomography and magnetic resonance imaging in the diagnosis of parotid tumors. Otolaryngol Head Neck Surg. 2003; 129:726–732. PMID: 14663442.

18. Shankland KR, Armitage JO, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2012; 380:848–857. PMID: 22835603.

19. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–242. PMID: 11807147.

20. Chernoff WG, Lampe HB, Cramer H, Banerjee D. The potential clinical impact of the fine needle aspiration/flow cytometric diagnosis of malignant lymphoma. J Otolaryngol. 1992; 21(Suppl 1):1–15.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download