Abstract
Giant cell tumor (GCT) of the craniofacial bones has been reported but they are not common. This tumor occurs more often in women than in men and predominantly affects patients around the third to fifth decade of life. GCTs are generally benign but can be locally aggressive as well. We report a case of GCT involving the temporomandibular joint (TMJ), which was initially thought to be temporomandibular disorder (TMD). A 22-year-old female presented with swelling and pain over the right temporal region for 18 months associated with jaw locking and clicking sounds. On examination, her jaw deviated to the right during opening and there was a 2×2 cm swelling over the right temporal region. Despite routine treatment for TMD, the swelling increased in size. Computed tomography and magnetic resonance imaging of the brain and TMJ revealed an erosive tumor of the temporal bone involving the TMJ which was displacing the temporal lobe. Surgical excision was done and the tumor removed completely. Histopathological examination was consistent with a GCT. No clinical or radiological recurrence was detected 10 months post-surgery.
Giant cell tumor (GCT) of bone usually occurs in the epiphyses of long bones like the distal femur, proximal tibia, distal radius and proximal humerus1. Craniofacial bone involvement is rare but has been reported to occur in the mandible, temporal bone, maxilla, occipital and sphenoid23. The incidence of GCT varies between regions and is highest amongst the Asian population, especially the Chinese and Japanese where they account for nearly 15% of all primary bone tumors4. GCTs affect women more than men at a ratio of 3:225. This tumor is mainly diagnosed during the third to fifth decade of life6. Although generally thought to be benign tumors, GCTs are known to be locally aggressive at times, with local recurrence occurring in about 25%-35% of patients3.
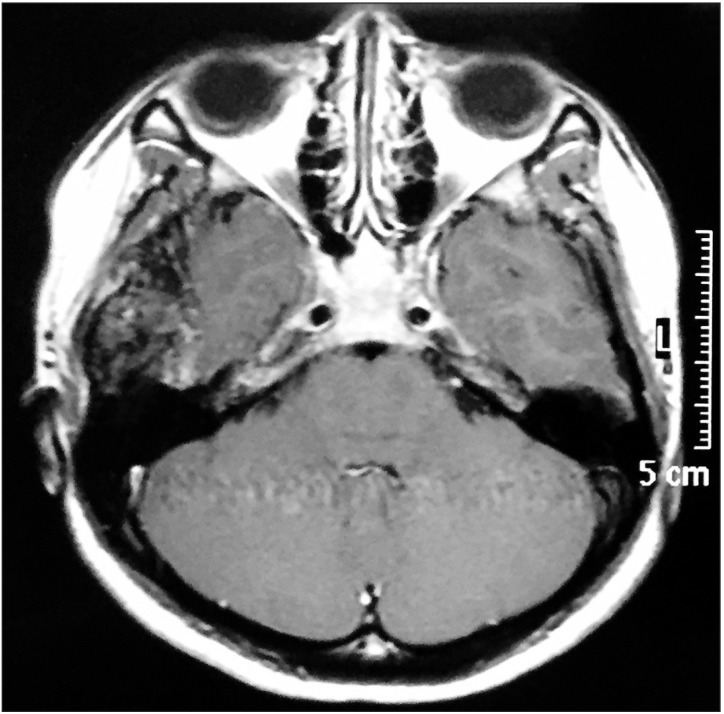
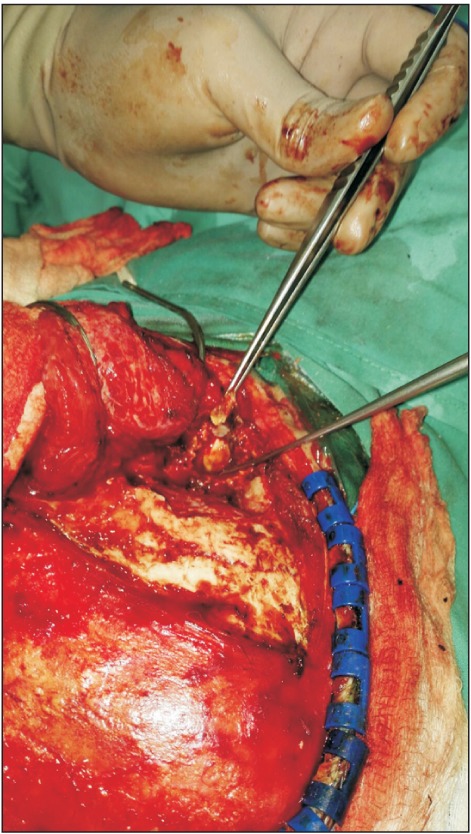
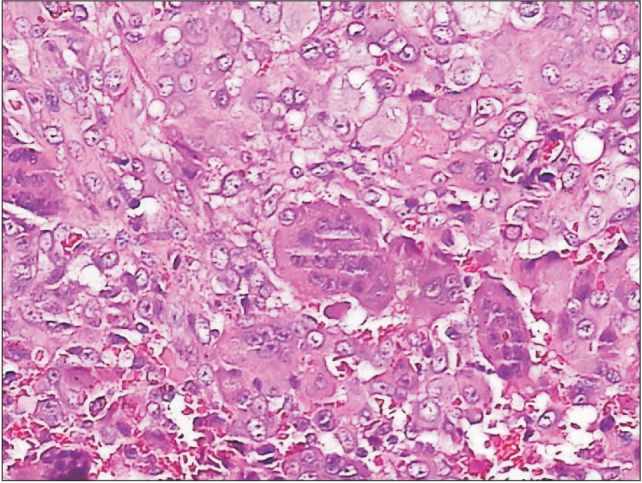
This is a case of a 22-year-old female who was previously well till she experienced swelling and pain over her right temporal region for 18 months. She had episodes of jaw locking and could hear clicking sounds when chewing or talking. Her hearing was slightly reduced on the right side. She sought medical help but was treated as temporomandibular disorder (TMD) and was given analgesics, physiotherapy and an occlusal splint. The pain reduced after the prescribed treatment and the swelling was inconspicuous initially but increased in size and hence further investigations were carried out. Examination revealed a 2×2 cm swelling over the right temporal region which was firm and tender on palpation. Upon opening her mouth, her mandible would deviate to the right side. Facial nerve and trigeminal nerve function was intact. Computed tomography (CT) scan showed an aggressive erosive tumor of the squamous temporal bone extending to the right temporomandibular joint (TMJ).(Fig. 1) An ultrasound-guided fine needle aspiration biopsy was performed. The result was suggestive of a xanthogranuloma. A magnetic resonance imaging (MRI) of the brain and TMJ was performed which showed an extra-axial mass at the right middle cranial fossa involving the right TMJ, measuring 4.2×1.6×2.6 cm. The mass was enhanced heterogeneously post-contrast and displaced the adjacent temporal lobe.(Fig. 2) During surgery, we used a modified frontotemporal flap for access to the temporal bone and TMJ. An intraoral right sulcular incision along the ascending ramus of the mandible was used for access to the coronoid process. Intraoperatively, the tumor was seen to invade the temporal bone, mandibular condyle, TMJ and overlying temporalis muscle but did not invade the temporal dura. The tumor was hard and yellowish in color. The patient underwent right partial temporal craniectomy, removal of part of the mandibular condyle and zygomatic arch, excision of the coronoid process, and excision of the TMJ.(Fig. 3) The zygomatic arch was reconstructed with titanium mesh. The patient recovered uneventfully after the surgery. Histopathological examination of the tumor revealed fibrous connective tissue with a few foci of numerous multinucleated giant cells, histiocytes, neutrophils, lymphocytes and occasional foam cells. There was peripheral bone and adjacent fibrocartilageneous tissue as well.(Fig. 4) A final diagnosis of benign GCT was made. No recurrence at 10 months post-surgery was detected.
GCTs are usually benign but have been known to be locally aggressive and occasionally metastasize, especially to the lung789. Very rarely, GCTs may turn into sarcoma4. The usual sites of occurrence are the epiphysis of long bones and less than 2% occur in the head and neck region where the usual sites are the sphenoid and temporal bones10. Less than 30 cases of GCT in the TMJ have been reported so far. Portions of the temporal bone form by endochondral ossification, which is the same way epiphyses of long bones are formed and thus it is possible that the temporal bone is more prone to develop GCTs because of this11. Patients usually present with progressive pain and swelling over the site. In the temporal region, hearing impairment and facial nerve paralysis can occur due to compression or local invasion from the tumor12. Involvement of the TMJ causes jaw locking, deviation of mandibular movement and clicking sounds. These three symptoms and signs are also common with TMD13. We would like to highlight the danger of treating patients as TMD before a precise diagnosis is made. A thorough history and examination should be done and a list of differential diagnoses should be considered, including tumors14. TMD may present with pain and swelling at the temporal area as in this case, but swelling in TMD is different and not common. Some patients may still have temporal swelling but the swelling should be softer on palpation and not persistent in size as compared to tumors. The swelling seen in TMD may be present during and after chewing and should decrease gradually between meals.
GCTs appear lytic, subarticular, eccentrically located and usually lack a sclerotic rim on radiographs. Local bony destruction, cortical breakthrough and soft tissue expansion may also be seen. CT will rarely provide information that helps physicians arrive at a diagnosis but may be useful in delineating tumor extent, evaluation of cortical integrity and determination of tumor recurrence15. On MRI, GCTs have low signal intensity on T1-weighted images, heterogeneous high signal intensity on T2-weighted images and heterogeneous enhancement with gadolinium. MRI is the preferred imaging modality for GCTs, as the diagnostic accuracy of MRI is high and it can detect soft tissue and intra-articular extension16.
Macroscopically, most GCTs are soft and fleshy and appear grey to light red or dark reddish-brown. There may be areas of cyst, hemorrhage or fibrous septa formation as well. The margins are usually ill defined, which explains the high percentage of recurrence if only curettage is done417.
GCT is a neoplasm of stromal-like neoplastic cells that are able to recruit macrophage and multinucleate osteoclast-like giant cells. Histologically, GCTs are characterized by the finding of large osteoclast-like multinucleated giant cells scattered among a background of plump or spindle shaped mononuclear stromal cells. The stromal cells may be mitotically active but should not have abnormal or atypical mitotic cells. These giant cells have approximately 10-20 nuclei per cell, but may have 100 or more nuclei. There may be reactive bone formation usually at the periphery and reactive changes such as reactive fibrosis, necrosis, hemorrhage and xanthogranulomatous inflammation. This is likely the reason the ultrasound-guided fine needle aspiration biopsy result showed a xanthogranuloma. GCTs often have abundance of neovascularization, which explains the hemorrhages that are frequently seen within such tumors418.
Important differential diagnoses of GCTs are osseous lesions that are giant cell rich. It is important to consider lesions such as giant cell reparative granuloma, hyperparathyroidism, non-ossifying fibroma, chondroblastoma, solid areas of aneurysmal bone cyst, malignant fibrous histiocytoma and osteogenic sarcoma4.
Surgery with the aim of wide excision is the mainstay of treatment for GCTs, preferably with a wide margin of normal tissue81619. However, total removal of the skull base and cranial GCTs is technically challenging. Radiotherapy is reserved for cases where wide excision cannot be achieved or for patients who are not fit for surgery. Irradiation-induced sarcomatous transformation is a known risk with orthovoltage radiation, but interestingly there is less risk with current use of megavoltage radiation19. Denosumab, a receptor activator of nuclear factor kappa-B ligand (RANKL) inhibitor has been approved for use in recurrent and unresectable GCTs20. High dose dexamethasone therapy had been used effectively to rapidly reduce the size of these tumors but unfortunately, discontinuation of steroids is associated with re-growth in nearly every case4.
References
1. Unni KK. Dahlin's bone tumors: general aspects and data on 11,087 cases. 5th ed. Philadelphia: Lippincott-Raven;1996. p. 263–285.
2. Schajowicz F. Tumors and tumor-like lesions of bone: pathology, radiology, and treatment. 2nd ed. Berlin: Springer-Verlag;1994. p. 257–295.
3. Bertoni F, Unni KK, Beabout JW, Ebersold MJ. Giant cell tumor of the skull. Cancer. 1992; 70:1124–1132. PMID: 1515987.

4. Zheng MH, Robbins P, Xu J, Huang L, Wood DJ, Papadimitriou JM. The histogenesis of giant cell tumour of bone: a model of interaction between neoplastic cells and osteoclasts. Histol Histopathol. 2001; 16:297–307. PMID: 11193206.
5. Huvos AG. Bone tumors: diagnosis, treatment and prognosis. Tumors of histiocytic or fibrohistiocytic origin. 2nd ed. Philadelphia: Saunders;1991. p. 429–467.
6. Robbins SL, Cotran RS, Kumar V. Robbins pathological basis of disease. 5th ed. Philadelphia: WB Saunders;1994. p. 1245–1246.
7. Vanel D, Contesso G, Rebibo G, Zafrani B, Masselot J. Benign giant-cell tumours of bone with pulmonary metastases and favourable prognosis. Report on two cases and review of the literature. Skeletal Radiol. 1983; 10:221–226. PMID: 6648560.
8. Leonard J, Gökden M, Kyriakos M, Derdeyn CP, Rich KM. Malignant giant-cell tumor of the parietal bone: case report and review of the literature. Neurosurgery. 2001; 48:424–429. PMID: 11220389.

9. Min BI, Park YW. A case of metastatic giant cell tumor arising in two-year old girl. J Korean Assoc Oral Maxillofac Surg. 1988; 14:82–87.
10. Lee HJ, Lum C. Giant-cell tumor of the skull base. Neuroradiology. 1999; 41:305–307. PMID: 10344520.

11. Morriss-Kay GM. Derivation of the mammalian skull vault. J Anat. 2001; 199:143–151. PMID: 11523816.

12. Findlay JM, Chiasson D, Hudson AR, Chui M. Giant-cell tumor of the middle cranial fossa. Case report. J Neurosurg. 1987; 66:924–928. PMID: 3572521.
13. Manfredini D, Guarda-Nardini L, Winocur E, Piccotti F, Ahlberg J, Lobbezoo F. Research diagnostic criteria for temporomandibular disorders: a systematic review of axis I epidemiologic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 112:453–462. PMID: 21835653.

14. Lee BK. Evidence-based practice in the treatment of temporomandibular disorders. J Korean Assoc Oral Maxillofac Surg. 2012; 38:263.

15. Wang CS, Lou JH, Liao JS, Ding XY, Du LJ, Lu Y, et al. Recurrence in giant cell tumour of bone: imaging features and risk factors. Radiol Med. 2013; 118:456–464. PMID: 22872452.

16. Purohit S, Pardiwala DN. Imaging of giant cell tumor of bone. Indian J Orthop. 2007; 41:91–96. PMID: 21139758.

17. Wysocki RW, Soni E, Virkus WW, Scarborough MT, Leurgans SE, Gitelis S. Is intralesional treatment of giant cell tumor of the distal radius comparable to resection with respect to local control and functional outcome. Clin Orthop Relat Res. 2015; 473:706–715. PMID: 25472928.

18. Silvers AR, Som PM, Brandwein M, Chong JL, Shah D. The role of imaging in the diagnosis of giant cell tumor of the skull base. AJNR Am J Neuroradiol. 1996; 17:1392–1395. PMID: 8871731.
19. Chen ZX, Gu DZ, Yu ZH, Qian TN, Huang YR, Hu YH, et al. Radiation therapy of giant cell tumor of bone: analysis of 35 patients. Int J Radiat Oncol Biol Phys. 1986; 12:329–334. PMID: 2420770.

20. Branstetter DG, Nelson SD, Manivel JC, Blay JY, Chawla S, Thomas DM, et al. Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res. 2012; 18:4415–4424. PMID: 22711702.

Fig. 1
Computed tomography scan showing an erosive tumor of squamous temporal bone and temporomandibular joint.
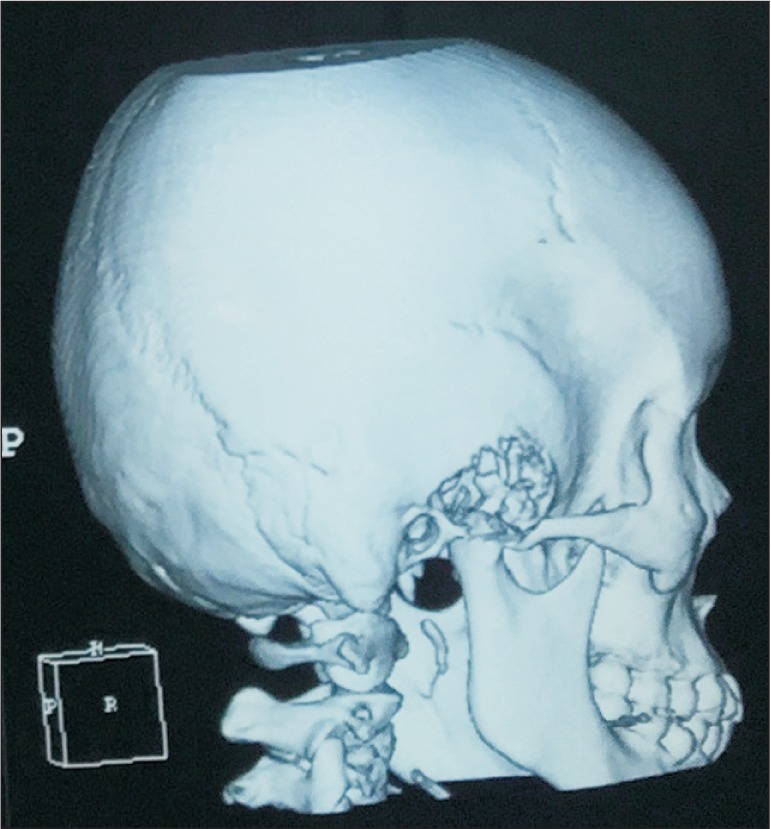
Fig. 2
Magnetic resonance imaging post-gadolinium showing an extra-axial heterogenous mass compressing on the right temporal lobe.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download