This article has been
cited by other articles in ScienceCentral.
Abstract
Osteonecrosis of the jaw (ONJ) is commonly described as an adverse effect of the use of bisphosphonates. A few cases of ONJ associated with tyrosine kinase inhibitors (sunitinib, imatinib) have been reported in the literature and usually they occurred in patients simultaneously treated with bisphosphonates. We report an atypical case of ONJ related only to imatinib. A 72-year-old male patient was treated with imatinib for metastases from gastrointestinal stromal tumors (GISTs). The patient developed ONJ after 22 months of imatinib only therapy. During his whole life, the patient had never been treated with bisphosphonates or radiotherapy. Microscope examination of the tissues confirmed the clinical diagnosis of diffuse osteonecrosis and showed absence of neoplastic cells. Thus, secondary localisations from GISTs were ruled out. Osteonecrosis of the lower jaw appeared 22 months after initial and exclusive therapy with imatinib. Therefore, imatinib monotherapy can induce ONJ in patients that have never been treated with bisphosphonates or radiotherapy.
Keywords: Imatinib mesylate, Osteonecrosis, Oral surgery, Gastrointestinal stromal tumors, Bone remodeling
I. Introduction
Imatinib mesylate is a member of a new class of chemotherapic agents that inhibit tyrosine kinase, a protein which belongs to a family of ubiquitous enzymes having a strategic role in signal transduction pathways and which influence gene transcription and/or DNA synthesis. Cell studies demonstrate that imatinib specifically inhibits proliferation of myeloid cell lines that express the BCR-ABL fusion proteins associated with chronic myeloid leukemia (CML). So, imatinib mesylate is used to treat CML, acute lymphoblastic leukemia and gastrointestinal stromal tumors (GISTs)
1.
Osteonecrosis of the jaw (ONJ), commonly described as an adverse effect of the use of bisphosphonates, is the progressive destruction and death of bone that affects the mandible or maxilla of patients exposed to treatment with nitrogen-containing bisphosphonates, in the absence of previous radiation treatment
2.
A few cases of ONJ associated with tyrosine kinase inhibitors (sunitinib, imatinib) have been reported in the literature and usually ONJ occurred in patients simultaneously treated with bisphosphonates. There are a lot of common side-effects of imatinib (nausea, vomiting, weakness, muscle cramps, edema especially periorbital and of the ankles, diarrhoea, exanthema, hypo-hyperpigmentation of the palate or oral mucosa) but no case of ONJ related to imatinib has been previously reported.
II. Case Report
A 72-year-old Caucasian male came to the Emergency Unit of Siena University Hospital (Siena, Italy) complaining of submandibular and right laterocervical pain with onset several days earlier. Medical history revealed that the patient had CD117-positive GISTs with a c-Kit genetic mutation since 2012. Since January 2013, he had been on imatinib at doses of 400 mg/day for 3 months followed by 600 mg/day for 4 months and then 800 mg/day. He had never taken bisphosphonates or undergone radiotherapy in the head and neck region. Moreover, he was not taking any other medication.
Examination, initially conducted by an ENT (ear-nose-throat) specialist, showed slight swelling at the right mandibular angle, multiple laterocervical and right submandibular lymphadenopathies, and hot reddened skin without signs of fistulas. Oral examination showed exposed bone in the right retromolar triangle, halitosis and sialorrhea.(
Fig. 1. A) Rhino-fibrolaryngoscopic examination did not detect irregularities or pathological processes in the pharyngeal and laryngeal regions. The ENT specialist referred the patient for dental examination.
Medical history included surgical removal of the distal root of the first lower molar 10 years earlier. On April 2014 the patient went to his dentist complaining of lower right quadrant toothache. The dentist confirmed the finding of the ENT specialist and also found mobility of the mandibular right first molar and anaesthesia/hypoesthesia of the right half of the lower lip, suggesting homolateral mandibular nerve compression.(
Fig. 1. B) The oral mucosa was normal. Since the patient did not recall exactly what dental work had been done, he consented to our contacting his dentist, who confirmed having performed a dental X-ray and tooth extraction (#47) because the tooth had fractured vertically and could not be saved.(
Fig. 2. A) The extraction was performed under block anaesthesia with articaine 1:100.000 (1.8 mL) and suture hemostatic control. The patient was prescribed 1 g amoxicillin and clavulanic acid every 12 hours for 6 days. When the stitches were removed on day 7, the wound appeared to have healed. Five weeks after the extraction, the patient had pain in the same region and halitosis but did not seek medical advice, preferring to take nonsteroidal anti-inflammatory drugs and the antibiotic again (1 g amoxicillin and clavulanic acid, every 12 hours). Since the pain did not resolve, a week later he presented at the emergency unit where ENT examination was carried out. The specialist ordered an X-ray of the dental arches (
Fig. 2. B) which showed sequestration of the right mandibular bone involving the retromolar triangle. An oral swab was taken and after disinfecting the oral cavity with 0.2% chlorexidine with anti-discoloration system, a bone fragment measuring 2.5×1.5 cm was removed together with underlying gingival tissue. The bone was sent to the pathology lab for examination. Cone-beam computed tomography (CBCT) was requested. The patient was prescribed antibiotics (3 g/day amoxicillin and clavulanic acid and 500 mg/day levofloxacin) and discharged.
The pathology results available 72 hours later indicated positivity for
Staphylococcus aureus,
Candida albicans,
Escherichia coli, and
Enterococcus faecalis. Since the antibiogram showed sensitivity to levofloxacin, the patient continued the therapy already prescribed, to which fluconazole was added for
C. albicans. CBCT showed a large area of osteonecrosis of the right hemimandibular body and angle with erosion of the vestibular cortex and complete destruction of the lingual cortex involving the mylohyoid line and the mandibular canal.(
Fig. 3) Microscope examination of the tissues confirmed the clinical diagnosis of diffuse osteonecrosis and absence of neoplastic cells, therefore secondary localisations from GIST were excluded.(
Fig. 4) Employing the Naranjo adverse drug reaction probability scale to determine the association of imatinib with osteonecrosis, the score revealed a probable adverse drug reaction.
III. Discussion
Imatinib is used to treat adults with GISTs that cannot be removed with surgery or have spread to other parts of the body, and adults who are at risk of GISTs coming back after surgical removal. The dose depends on the disease being treated, the age and condition of the patient, and the response to treatment, but it should not exceed 800 mg a day. The patient had been treated only with imatinib for 22 months at doses of 400 mg/day for 3 months followed by 600 mg/day for 4 months and then 800 mg/day and never with bisphosphonates or radiotherapy. In line with indications in the literature
34, the oncologist did not suspend imatinib therapy because the GISTs were metastases. Clinical course after the tooth extraction included apparent healing in the first weeks followed by rapid local worsening leading to bone sequestration with pain, swelling, halitosis and partial hypoesthesia of the lower lip. This evolution largely resembles the clinical course of osteonecrosis due to bisphosphonates
2. Although the patient had never been treated with bisphosphonates or radiotherapy of the head or neck, his clinical picture was identical to osteonecrosis stage 2 subclass b due to bisphosphonates. Therapy with imatinib and the concomitant dental extraction seem likely causes of the resulting osteonecrosis.
Only one case of osteonecrosis involving fracture of the tibia
5 has been reported in a patient on imatinib. Various cases of osteonecrosis of the mandible have been reported in patients taking imatinib and bisphosphonates
6. In our case, the patient was taking only imatinib.
Other tyrosine-kinase inhibitors, usually sunitinib, have been associated with an increased risk for ONJ in patients receiving concomitant treatment with intravenous bisphosphonates
7. Cases of ONJ have also been reported in patients with renal cell carcinoma treated with sunitinib monotherapy
8. Sunitinib is a vascular endothelial growth factor (VEGF) receptor 1-3 antagonist: inhibition of VEGF-dependent angiogenesis may play a major role in the onset of sunitinib-associated ONJ
9. The present case of ONJ, however, cannot be due solely to a similar mechanism, since imatinib does not block VEGF receptors, although it could partly reduce VEGF expression through inhibition of the platelet-derived growth factor receptor (PDGFR) or c-Kit
1011.
Some evidence suggests that imatinib can directly target skeletal cells, leading to dysregulation of bone remodelling
12. Inhibition of c-Fms and c-Kit signaling by imatinib may decrease osteoclast number and activity. Imatinib also inhibits the proton-generating activity of carbonic anhydrase II, preventing dissolution of minerals from bone and hence inhibiting bone resorption. Furthermore, inhibition of PDGFR signalling on osteoblasts can reduce the production of osteoclastogenic cytokines, including macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), and inhibit osteoblastic cell proliferation.
A few cases of ONJ associated with tyrosine kinase inhibitors have been reported in the literature and they always occurred in patients simultaneously treated with bisphosphonates.
Considering the clinical and pharmacological history of our patient, ONJ could have been caused by imatinib treatment. Finally, it would be a good practice to avoid tooth extraction during imatinib therapy.
Figures and Tables
Fig. 1
First evaluation of the patient suffering by gastrointestinal stromal tumors and treated for 22 months with imatinib. A. Oral examination showed exposed bone in the right retromolar triangle, halitosis, sialorrhea, and anaesthesia. B. A black line restricts the region affected by anaesthesia.

Fig. 2
Radiologic images compare the situation before and after extraction of tooth (#47) during imatinib therapy. A. Panoramic radiograph before extraction of tooth (#47). B. Panoramic radiograph after extraction showing bone sequestration and osteonecrosis in the mandibular right retromolar triangle.

Fig. 3
Three-dimensional cone-beam computed tomography reconstruction of the jaw during imatinib therapy. A. Vestibular view of the jaw shows the perforation of mandibular bone cortex in the patient treated only with imatinib for 22 months. B. Lingual view of the jaw shows the destruction of normal anatomy and erosion of the mandibular canal.
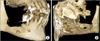
Fig. 4
Histologic examination of the tissues confirmed the clinical diagnosis of diffuse osteonecrosis of the jaw. A. Histologic section of bone fragment showing diffuse osteonecrosis (H&E staining, ×25). Given the absence of neoplastic cells, secondary localisations from gastrointestinal stromal tumors were excluded. B. Histologic section of gingival fragment showing chronic purulent exudative inflammation, granulation tissue and acanthosis of the superficial layer (H&E staining, ×50).

Acknowledgements
All authors thank the patient who has kindly given his consent to collect material for this study.
References
1. Dulucq S, Krajinovic M. The pharmacogenetics of imanitib. Genome Med. 2010; 2:85.

2. Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, et al. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg. 2014; 72:1938–1956.

3. Lutz JC, El-Bouihi M, Vidal N, Fricain JC, Robert M, Deminière C, et al. Mandibular metastases from an ileum stromal tumor. Rev Stomatol Chir Maxillofac. 2008; 109:399–402.

4. Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001; 344:1052–1056.

5. Yeh CN, Fu CJ, Yen TC, Chiang KC, Jan YY, Chen MF. Osteonecrosis of the tibia associated with imatinib in metastatic GI stromal tumor. J Clin Oncol. 2013; 31:e248–e250.

6. Nicolatou-Galitis O, Razis E, Galiti D, Vardas E, Tzerbos F, Labropoulos S. Osteonecrosis of the jaw in a patient with chronic myelogenous leukemia receiving imatinib. A case report with clinical implications. Forum Clin Oncol. 2013; 4:29–33.
7. Beuselinck B, Wolter P, Karadimou A, Elaidi R, Dumez H, Rogiers A, et al. Concomitant oral tyrosine kinase inhibitors and bisphosphonates in advanced renal cell carcinoma with bone metastases. Br J Cancer. 2012; 107:1665–1671.

8. Fleissig Y, Regev E, Lehman H. Sunitinib related osteonecrosis of jaw: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:e1–e3.

9. Abramson RG, Abramson VG, Chan E, Horn L, Keedy VL, Pao W, et al. Complications of targeted drug therapies for solid malignancies: manifestations and mechanisms. AJR Am J Roentgenol. 2013; 200:475–483.

10. Vlahovic G, Rabbani ZN, Herndon JE 2nd, Dewhirst MW, Vujaskovic Z. Treatment with Imatinib in NSCLC is associated with decrease of phosphorylated PDGFR-beta and VEGF expression, decrease in interstitial fluid pressure and improvement of oxygenation. Br J Cancer. 2006; 95:1013–1019.

11. Litz J, Krystal GW. Imatinib inhibits c-Kit-induced hypoxiainducible factor-1alpha activity and vascular endothelial growth factor expression in small cell lung cancer cells. Mol Cancer Ther. 2006; 5:1415–1422.

12. Vandyke K, Fitter S, Dewar AL, Hughes TP, Zannettino AC. Dysregulation of bone remodeling by imatinib mesylate. Blood. 2010; 115:766–774.









 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download