Abstract
Osteoid osteomas are benign skeletal neoplasms that are commonly encountered in the bones of the lower extremities, but are exceedingly rare in jaw bones with a prevalence of less than 1%. This unique clinical entity is usually seen in younger individuals, with nocturnal pain and swelling as its characteristic clinical manifestations. The size of the lesion is rarely found to be more than 2 cm. We hereby report a rare case of osteoid osteoma originating from the neck of the mandibular condyle that grew to large enough proportions to result in conductive hearing loss in addition to pain, swelling and restricted mouth opening. In addition, an effort has been made to review all the documented cases of osteoid osteomas of the jaws that have been published in the literature thus far.
Osteoid osteoma is a distinct clinical entity that was first described by Jaffe1 in 1935. It is a benign skeletal neoplasm that is usually encountered in young adults under the age of 30. The most classical feature of the tumor is pain accompanied by vasomotor disturbances. Its prevalence in jaws is less than 1%, making this unique entity exceeding rare in the jaw bones2. Furthermore, only five cases in jaws are reported so far in the literature, wherein the osteoid osteoma has always occurred in the condylar region of the mandible. Among all the documented cases in the literature, the authors Yang and Qiu3 reported the largest osteoid osteoma (4.0×3.5 cm) that presented in the left articular eminence of a 24-year-old female. We present an exceedingly large osteoid osteoma in the mandibular condylar neck (7.0×6.5 cm) that presented in a 41-year-old female, causing conductive hearing loss and restricted mouth opening in addition to pain. In the scientific literature, we did not come across any cases with clinical manifestations similar to our case or with such a large size osteoid osteoma. In addition, all the cases that are documented in the scientific literature regarding osteoid osteomas presenting in the jaws were also reviewed456789101112.(Table 1)
The written informed consent was obtained from the patients.
A 41-year-old female patient was referred to our unit with the chief complaint of swelling in the right preauricular region over 7 years.(Fig. 1. A) The increase in the size of the swelling was slow but steady over these 7 years. Within a period of 4 months from the development of swelling, she started experiencing pain in the same region that was dull and intermittent, which increased in intensity at night and was relieved with analgesics. There was also a positive history of decreased hearing in the right ear and restricted mouth opening for 3 months. The medical history of the patient was not contributory in any manner. On extra-oral examination, a well-defined swelling was seen extending superiorly from a line joining the tragus of the right ear to the lateral canthus, to about 2 cm form the lower border of the mandible inferiorly and posteroanteriorly from the right ear lobule to about 2.5 cm anterior to it.(Fig. 1. B) On palpation, the swelling was bony hard in consistency, non-tender and attached to the underlying bone. The skin overlying the swelling was of normal color and was movable with no local rise in temperature. The regional lymph nodes were found to be non-palpable. The mouth opening was found to be 21 mm.(Fig. 1. C) The intraoral examination did not reveal any abnormalities.(Fig. 1. D)
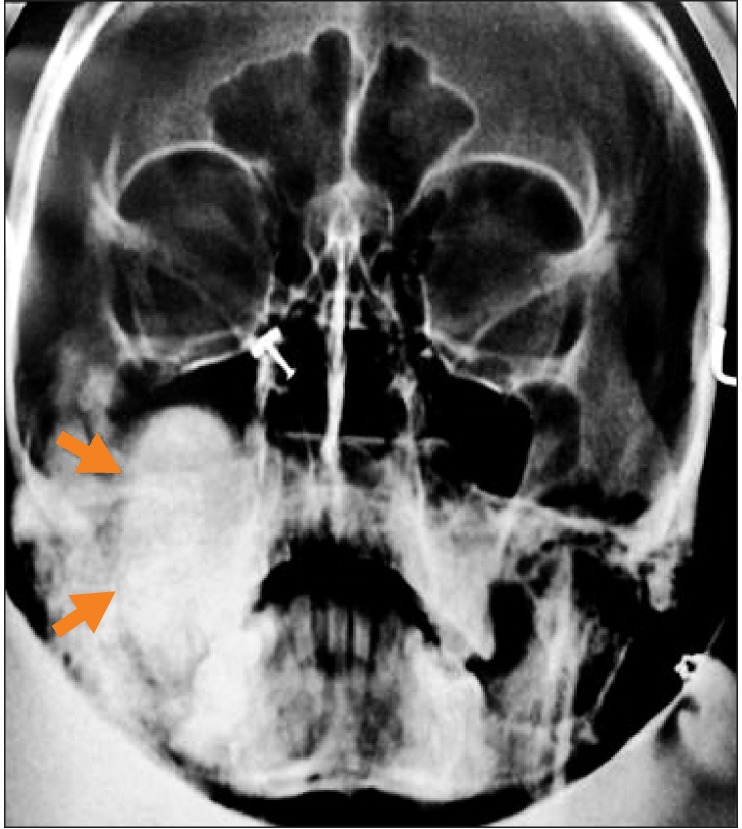
The patient was initially subjected to radiographic imaging in the form of the Waters projection, revealing a well-defined radioopacity with a thin radioluscent rim in relation to right mandibular condyle, roughly measuring roughly about 6.0×3.0 cm in its greatest dimensions.(Fig. 2) In order to define the lesion size more precisely in order to assist in surgical planning, a high resolution helical computed tomographic (CT) scan was sought. The image revealed a large, lobulated and heterogeneously ossified lesion of size 7.0×6.8×6.4 cm, originating from the right mandibular condylar neck and extending into the right infratemporal fossa and masticator space.(Fig. 3. A-D) Sclerotic changes were noted in the upper portion of the ramus of the mandible. A well circumscribed circular nidus on the buccal aspect of the condylar neck with decreased attenuation was visible with a variable amount of surrounding sclerosis and periosteal new bone formation.(Fig. 3. A) The exact extent of the lesion was as follows. Anteriorly, the lesion was indenting the posterior wall of the right maxillary sinus, causing bowing of the posterior sinus wall. (Fig. 3. E) Posteriorly, the lesion extended up to the mastoid process with subsequent displacement of the internal carotid artery and internal jugular vein. Medially, the mass obliterated the right parapharyngeal space through compression of the right pterygopalatine fossa and right pterygoid bone. Compression of the Eustachian tube was also noted. Laterally, the lesion extended up to the subcutaneous plane, indenting the parotid gland. Superiorly, the lesion indented the greater wing of the sphenoid through mild intracranial extension into the right middle cranial fossa.(Fig. 3. F) Inferiorly, the lesion was seen to extend up to the upper third of the ramus of the mandible. Based on radiographic images, a differential diagnosis of fibro-osseous lesion, osteoblastoma, osteoid osteoma, fibro-osseous lesion, ossifying fibroma and giant cell granuloma was made. No similar lesions were found in other bones, including the mandible, on general physical and radiological examinations. Opinions from an ear nose throat (ENT) surgeon were sought regarding the patient's decreased hearing. Based on the Weber test and audiometry testing coupled with CT scans, the patient was diagnosed as having conductive hearing loss secondary to pressure compression of the Eustachian tube.
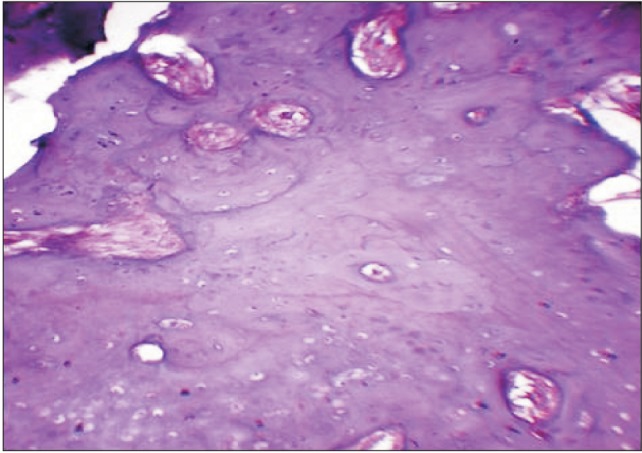
Routine haematological and bieochemical investigations showed no abnormalities. Considering the large dimensions of the lesion, an incisional biopsy of the lesion was planned through a preauricular incision, and a wedge of specimen was submitted for histopathological examination. The histopathologic picture was consistent with the diagnosis of osteoid osteoma.(Fig. 4) After obtaining written informed consent, the patient was scheduled for surgical excision of the lesion under general anesthesia. A modified Blair's incision was used to provide surgical access to the lesion.(Fig. 5. A) The mandibular ramus was horizontally osteotomized and the entire lesion was skeletonized from adjacent structures and delivered in toto. Clinically, the excised specimen measured roughly 7.0×6.5 cm in its greatest dimensions.(Fig. 5. B) The standard protocol of antibiotics and analgesics was followed. The excised specimen was once again submitted for histopathological examination, proving beyond doubt that the lesion was indeed osteoid osteoma.
The postoperative recovery was uneventful with a cosmetically acceptable scar. No recurrence was observed over a 1-year follow-up on clinical and radiological examinations. (Fig. 6, 7, 8) A gradual improvement to normal levels in hearing was seen on the affected side over a two month postoperative period. The improvement was confirmed by the Weber test and audiometry. There was definite improvement in mouth opening following surgery, with deviation of the mandible on the same side due to unopposed action of the contralateral lateral pterygoid muscle. The symptoms of jaw pain disappeared following excision of the tumorous mass. The patient will soon be scheduled for temporomandibular joint reconstruction to correct hollowness in the right preauricular region that had developed post-surgery.
Osteoid osteoma is defined by Lichtenstein as a “small, oval or roundish tumor like nidus that is predominantly composed of osteoid and trabeculae of newly formed bone deposited within the substratum of highly vascularized osteogenic connective tissue”4. To date, the true nature of such a lesion is not clear and therefore varied pathogenesis has been suggested, ranging from neoplastic to inflammatory reaction to unusual healing12.
Distinct benign skeletal neoplasms comprise 10% to 12% of all benign bone tumors and are usually seen in patients under 30 years of age. Males are affected three times as frequently as females. Osteoid osteomas have the predilection of occurring anywhere in the skeleton; the lower extremities are the most common sites, with 50% to 60% of the cases reported in the femur and tibia1113. However, it is seldom reported in jaw bones. As of now, only 26 cases of osteoid osteoma involving jaw bones have been documented in the literature. (Table 1) By analyzing the reported cases, we inferred that the majority of osteoid osteoma cases in jaws occurred during the second and third decades of life (n=16, 61.53%), with a slight female predilection (female to male ratio of 1.08:1). The reports clearly show that the mandible is more commonly involved than the maxilla (n=22, 84.61%), with the body region of the mandible being the most commonly affected site. As of now, only 5 cases of osteoid osteomas originating from the condylar region are reported in the literature, making the condylar region of the mandible a rare site for osteoid osteomas. To the best of our knowledge, an osteoid osteoma originating from the condylar neck has not yet been reported in the scientific literature. Pain was the most common presenting complaint in the reported cases (n=17, 65.38%).
In this study, we have put aside the norms set by other authors with regard to osteoid osteoma size. Most authors are of the opinion that this lesion usually does not grow more than 2 cm in size7812. However, we have presented a case wherein the osteoid osteoma grew to almost 3.5 time (7.0×6.5 cm) this standard size, making it the first case to be reported in the literature with such large dimensions.
Pain is the most frequent clinical symptom, which is unique to this tumor. The pain is dull or intermittent, characteristically worsens at night and is relieved via non-steroidal anti-inflammatory drugs (NSAIDs). This finding was also present in our case. Various theories have been put forward to explain the precise nature of the pain and its tendency to worsen at night. Some authors believe that prostaglandins, especially prostaglandin E2, plays a pivotal role; this is evident by the high concentration of prostaglandins that is found in the nidus of the lesion10. This further explains why the pain is alleviated following the ingestion of NSAIDs. Others are of the view that its rich vascularity brings about innervation of the free nerve endings or direct irritation of the nerve fibers, leading to pain and tenderness8.
The radiographic features of such a lesion further assist in diagnosis. Characteristically, there exists a central nidus that is less than 2 cm in diameter with a sclerotic bony margin. The zone of sclerosis surrounding the nidus is not included in the measurement, as it is considered to be a secondary reaction and may be extensive34. According to Jaffe1, the nidus was more radioluscent than radiopaque and is surrounded by reactive radioopacity, which was extended at variable distances from the nidus. Less mature lesions were more likely to have a radiopaque nidus, whereas fully mature osteoid osteomas had a radiolucent nidus. According to Pritchard and Mckay14, calcification of the osteoid in the later developmental stages resulted in a central opaque body that varied in density as calcification progressed. In the present case, a well-defined radioopaque mass with a thin radioluscent rim was observed. Non-specific findings on plain radiographs prompted surgeons to opt for additional imaging modalities. CT scans are currently the best radiographic examination for diagnosis of an osteoid osteoma; this is because they show more detail regarding the relationship between the tumor and adjacent structures in comparison to conventional radiography. Furthermore, sclerotic change of the adjacent bone and periosteal reaction is also well demonstrated. In our case, the osteoid osteoma was diagnosed using CT scans, which not only disclosed the exact dimensions and extent of the lesion, but also enabled better surgical planning. It is important to rule out Gardner syndrome in patients with osteomas. This syndrome is an autosomal dominant trait characterized by multiple osteomas, gastrointestinal polyps, skin and soft tissue tumors and multiple impacted or supernumerary teeth11.
From the histopathologic perspective, three distinct stages of ossification are described by Huvos49. In the initial stage, densely packed and actively proliferating osteoblasts are seen in a highly vascularized stroma. The intermediate stage is characterized by osteoid deposition between the osteoblasts. In the mature stage, the osteoid transforms into well-calcified compact trabeculae of an atypical bone, which are typically neither woven nor lamellar.
From the clinical and radiographic perspectives, the differential diagnosis can be osteoblastoma, fibro-osseous lesion, ossifying fibroma, or giant cell granuloma. Considering the large size of the osteoid osteoma in the present case and its similar presentation to osteoblastoma, it becomes challenging to differentiate these two clinical entities1516. Osteoblastomas can be asymptomatic or can cause significant pain and swelling, but the pain does not worsen at night and is not relieved via NSAIDs, which is typical of osteoid osteomas41217. Histologically, osteoid osteomas have a nidus composed of osteoid tissue surrounded by densely reactive bone (not seen in osteoblastoma), lack multinucleated giant cells and present less vascularization compared to osteoblastomas10. Fibrous dysplasia is a poorly defined lesion and has radioluscent and ground glass appearances in the early and mature stages, respectively, both of which were not seen in this case. Although ossifying fibromas have some features identical to osteoid osteomas, including both clinically and radiographically, they are usually painless with no nidus12. Giant cell granulomas are distinguished on the basis of multinucleated giant cells lying on extremely vascular stroma. This finding was not present in the histopathologic sections of the present case10.
The recommended treatment for such a tumor is complete excision, which alleviates the pain and cures the disease5. Although some reported cases have spontaneously regressed, identifying such cases in advance is difficult51819. Recurrences are rare following complete removal of the lesion, but some cases have been reported. Very rarely, the tumor can undergo sarcomatous or locally malignant changes12. As of now, only a single case of malignant transformation of osteoid osteoma into low grade aggressive osteoblastoma has been reported20. Long term follow-up is therefore recommended to detect locally malignant or sarcomatous changes.
In conclusion, we have presented a rare case of osteoid osteoma originating from the right condylar neck with very large proportions, causing conductive hearing loss in addition to pain, swelling and restricted mouth opening. To the best of our knowledge, an osteoid osteoma of such large dimensions has not been reported in the literature and therefore requires special mention. Furthermore, such large dimensions of the tumor make it increasingly difficult to distinguish osteomas from osteoblastomas due to the considerable overlap between both entities in clinical, histopathologic and radiographic features. Detailed clinical and radiographic imaging modalities are necessary to arriving at a diagnosis. The role of CT scans is instrumental in identifying the extent, dimensions and relationship to adjacent anatomical structures, in addition to identifying the number of lesions, sclerotic changes of the adjacent bone and periosteal reaction. Following surgical excision, a long term follow up is mandatory.
References
1. Jaffe HL. Osteoid osteoma: a benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg. 1935; 31:709–728.
2. Ida M, Kurabayashi T, Takahashi Y, Takagi M, Sasaki T. Osteoid osteoma in the mandible. Dentomaxillofac Radiol. 2002; 31:385–387. PMID: 12424638.

3. Yang C, Qiu WL. Osteoid osteoma of the eminence of the temporomandibular joint. Br J Oral Maxillofac Surg. 2001; 39:404–406. PMID: 11601826.

4. Singh A, Solomon MC. Osteoid osteoma of the mandible: a case report with review of the literature. J Dent Sci. 2012; DOI: 10.1016/j.jds.2012.10.002. [Epub ahead of print].

5. An SY, Shin HI, Choi KS, Park JW, Kim YG, Benavides E, et al. Unusual osteoid osteoma of the mandible: report of case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116:e134–e140. PMID: 23849381.

6. Foss EL, Dockerty MB, Good CA. Osteoid osteoma of the mandible; report of a case. Cancer. 1955; 8:592–594. PMID: 14379148.
7. Gupta OP, Jain RK, Agarwal MK, Khanna S, Shrivastava A. Osteoid osteoma of the mandible. Ear Nose Throat J. 1985; 64:206. 208. PMID: 3996270.
8. Tochihara S, Sato T, Yamamoto H, Asada K, Ishibashi K. Osteoid osteoma in mandibular condyle. Int J Oral Maxillofac Surg. 2001; 30:455–457. PMID: 11720052.

9. Chaudhary M, Kulkarni M. Osteoid osteoma of the mandible. J Oral Maxillofac Pathol. 2007; 11:52–55.
10. Rahsepar B, Nikgoo A, Fatemitabar SA. Osteoid osteoma of subcondylar region: case report and review of the literature. J Oral Maxillofac Surg. 2009; 67:888–893. PMID: 19304051.

11. Karandikar S, Thakur G, Tijare M, Shreenivas K, Agrawal K. Osteoid osteoma of mandible. BMJ Case Rep. 2011; 2011:bcr1020114886.

12. Mohammed I, Jannan NA, Elrmali A. Osteoid osteoma associated with the teeth: unusual presentation. Int J Oral Maxillofac Surg. 2013; 42:298–302. PMID: 22658497.

13. Barei DP, Moreau G, Scarborough MT, Neel MD. Percutaneous radiofrequency ablation of osteoid osteoma. Clin Orthop Relat Res. 2000; (373):115–124.

14. Pritchard JE, Mckay JW. Osteoid osteoma. Can Med Assoc J. 1948; 58:567–575. PMID: 18862254.
15. Alvares Capelozza AL, Gião Dezotti MS, Casati Alvares L, Negrão Fleury R, Sant'Ana E. Osteoblastoma of the mandible: systematic review of the literature and report of a case. Dentomaxillofac Radiol. 2005; 34:1–8. PMID: 15709098.

16. Rawal YB, Angiero F, Allen CM, Kalmar JR, Sedghizadeh PP, Steinhilber AM. Gnathic osteoblastoma: clinicopathologic review of seven cases with long-term follow-up. Oral Oncol. 2006; 42:123–130. PMID: 16129654.

17. Jones AC, Prihoda TJ, Kacher JE, Odingo NA, Freedman PD. Osteoblastoma of the maxilla and mandible: a report of 24 cases, review of the literature, and discussion of its relationship to osteoid osteoma of the jaws. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:639–650. PMID: 17052641.

18. Goldberg VM, Jacobs B. Osteoid osteoma of the hip in children. Clin Orthop Relat Res. 1975; (106):41–47.

19. Sim FH, Dahlin CD, Beabout JW. Osteoid-osteoma: diagnostic problems. J Bone Joint Surg Am. 1975; 57:154–159. PMID: 1112841.
20. Pieterse AS, Vernon-Roberts B, Paterson DC, Cornish BL, Lewis PR. Osteoid osteoma transforming to aggressive (low grade malignant) osteoblastoma: a case report and literature review. Histopathology. 1983; 7:789–800. PMID: 6629344.

Fig. 1
Preoperative photographs of the patient. A. Frontal view. B. Lateral view with clinical extensions of the lesion. C. Photograph depicting restricted mouth opening. D. Intraoral view.
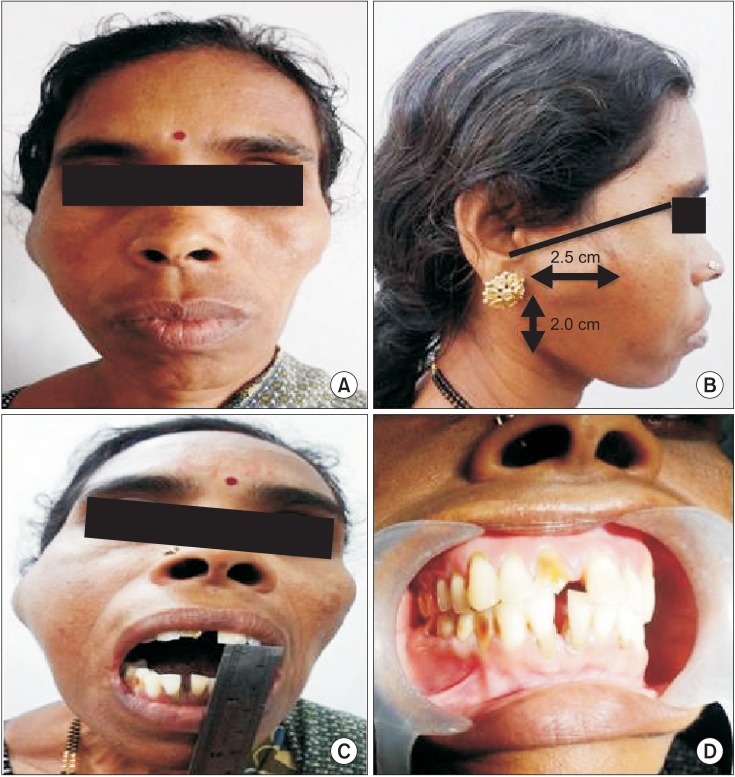
Fig. 3
Computed tomographic (CT) views of the case (plain and contrast enhanced). A. CT view showing the origin of the lesion from the neck of the condyle (arrow 1), nidus (arrow 2), and extent of the lesion (arrow 3 and 4). B. Origin of the lesion from the condylar neck (black arrow). C. Origin of the lesion from the condylar neck (black arrow). D. Contrast enhanced view showing the dimensions of the lesion (2D). E. View showing indentation of the posterior maxillary sinus wall (black arrow). F. View showing lesion extension intracranially into the right middle cranial fossa (black arrow).
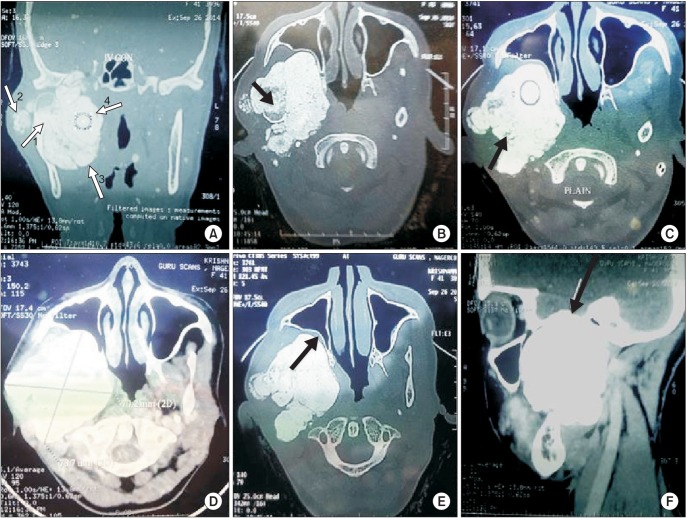
Fig. 5
Intraoperative photographs. A. Incision planning and exposure of the lesion. B. Excised specimen in toto.
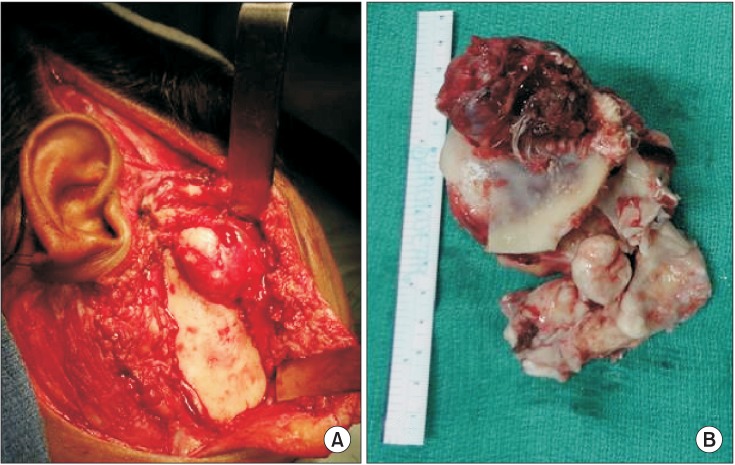
Fig. 6
Postoperative clinical photographs at 1 year. A. Frontal view. B. Profile view showing the hollowness in the right temporomandibular joint region and a cosmetically acceptable scar. C. Photograph depicting improvement in mouth opening.
Fig. 8
Postoperative plain computed tomographic (CT) scan at 1 year with three-dimensional (3D) reconstructive images showing no recurrence. A-C. CT views depicting no recurrence. D-F. 3D reconstructive images.
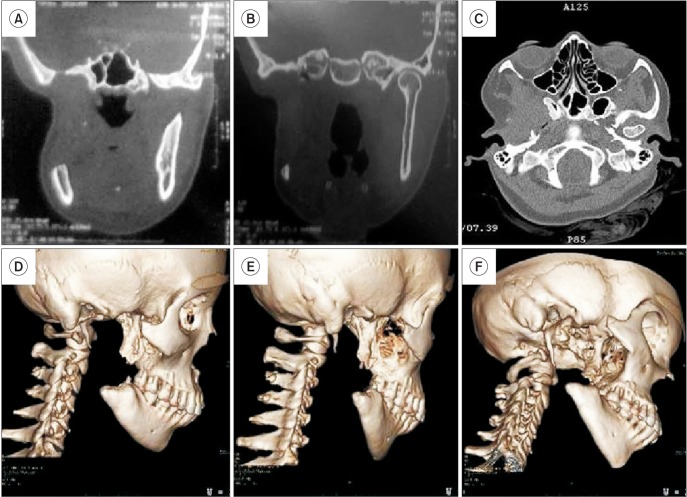
Table 1
Documented cases of osteoid osteoma in the jaws published in the scientific literature thus far
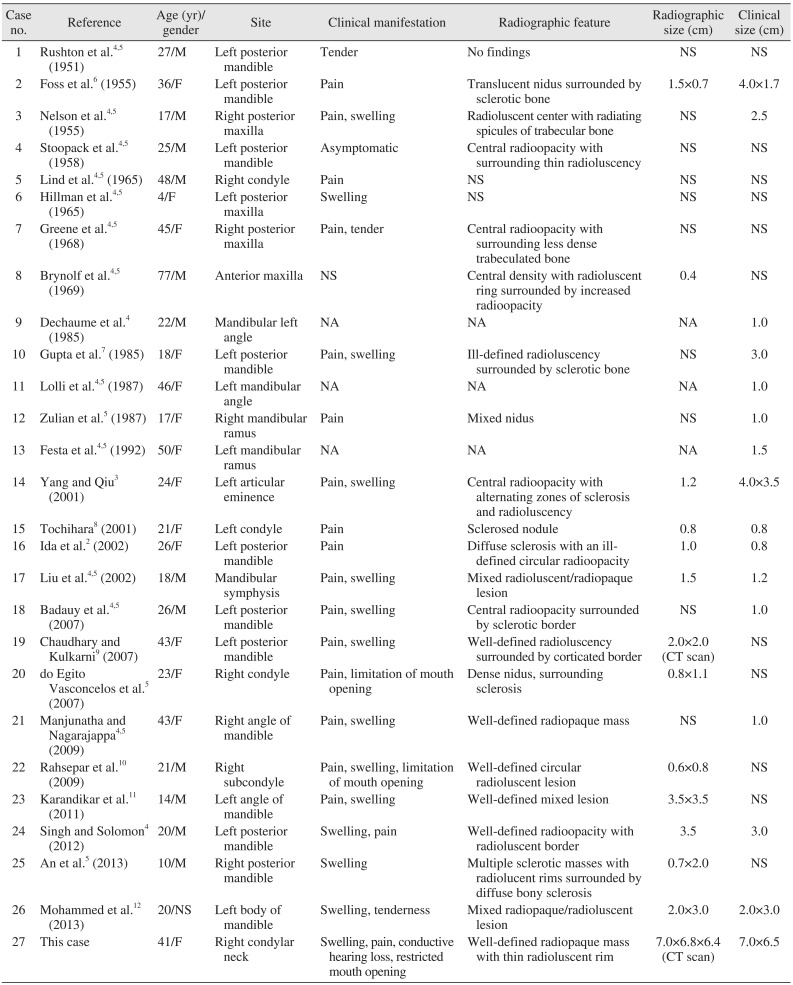
| Case no. | Reference | Age (yr)/ gender | Site | Clinical manifestation | Radiographic feature | Radiographic size (cm) | Clinical size (cm) |
|---|---|---|---|---|---|---|---|
| 1 | Rushton et al.4,5 (1951) | 27/M | Left posterior mandible | Tender | No findings | NS | NS |
| 2 | Foss et al.6 (1955) | 36/F | Left posterior mandible | Pain | Translucent nidus surrounded by sclerotic bone | 1.5×0.7 | 4.0×1.7 |
| 3 | Nelson et al.4,5 (1955) | 17/M | Right posterior maxilla | Pain, swelling | Radioluscent center with radiating spicules of trabecular bone | NS | 2.5 |
| 4 | Stoopack et al.4,5 (1958) | 25/M | Left posterior mandible | Asymptomatic | Central radioopacity with surrounding thin radioluscency | NS | NS |
| 5 | Lind et al.4,5 (1965) | 48/M | Right condyle | Pain | NS | NS | NS |
| 6 | Hillman et al.4,5 (1965) | 4/F | Left posterior maxilla | Swelling | NS | NS | NS |
| 7 | Greene et al.4,5 (1968) | 45/F | Right posterior maxilla | Pain, tender | Central radioopacity with surrounding less dense trabeculated bone | NS | NS |
| 8 | Brynolf et al.4,5 (1969) | 77/M | Anterior maxilla | NS | Central density with radioluscent ring surrounded by increased radioopacity | 0.4 | NS |
| 9 | Dechaume et al.4 (1985) | 22/M | Mandibular left angle | NA | NA | NA | 1.0 |
| 10 | Gupta et al.7 (1985) | 18/F | Left posterior mandible | Pain, swelling | Ill-defined radioluscency surrounded by sclerotic bone | NS | 3.0 |
| 11 | Lolli et al.4,5 (1987) | 46/F | Left mandibular angle | NA | NA | NA | 1.0 |
| 12 | Zulian et al.5 (1987) | 17/F | Right mandibular ramus | Pain | Mixed nidus | NS | 1.0 |
| 13 | Festa et al.4,5 (1992) | 50/F | Left mandibular ramus | NA | NA | NA | 1.5 |
| 14 | Yang and Qiu3 (2001) | 24/F | Left articular eminence | Pain, swelling | Central radioopacity with alternating zones of sclerosis and radioluscency | 1.2 | 4.0×3. |
| 15 | Tochihara8 (2001) | 21/F | Left condyle | Pain | Sclerosed nodule | 0.8 | 0.8 |
| 16 | Ida et al.2 (2002) | 26/F | Left posterior mandible | Pain | Diffuse sclerosis with an ill-defined circular radioopacity | 1.0 | 0.8 |
| 17 | Liu et al.4,5 (2002) | 18/M | Mandibular symphysis | Pain, swelling | Mixed radioluscent/radiopaque lesion | 1.5 | 1.2 |
| 18 | Badauy et al.4,5 (2007) | 26/M | Left posterior mandible | Pain, swelling | Central radioopacity surrounded by sclerotic border | NS | 1.0 |
| 19 | Chaudhary and Kulkarni9 (2007) | 43/F | Left posterior mandible | Pain, swelling | Well-defined radioluscency surrounded by corticated border | 2.0×2.0 (CT scan) | NS |
| 20 | do Egito Vasconcelos et al.5 (2007) | 23/F | Right condyle | Pain, limitation of mouth opening | Dense nidus, surrounding sclerosis | 0.8×1.1 | NS |
| 21 | Manjunatha and Nagarajappa4,5 (2009) | 43/F | Right angle of mandible | Pain, swelling | Well-defined radiopaque mass | NS | 1.0 |
| 22 | Rahsepar et al.10 (2009) | 21/M | Right subcondyle | Pain, swelling, limitation of mouth opening | Well-defined circular radioluscent lesion | 0.6×0.8 | NS |
| 23 | Karandikar et al.11 (2011) | 14/M | Left angle of mandible | Pain, swelling | Well-defined mixed lesion | 3.5×3.5 | NS |
| 24 | Singh and Solomon4 (2012) | 20/M | Left posterior mandible | Swelling, pain | Well-defined radioopacity with radioluscent border | 3.5 | 3.0 |
| 25 | An et al.5 (2013) | 10/M | Right posterior mandible | Swelling | Multiple sclerotic masses with radiolucent rims surrounded by diffuse bony sclerosis | 0.7×2.0 | NS |
| 26 | Mohammed et al.12 (2013) | 20/NS | Left body of mandible | Swelling, tenderness | Mixed radiopaque/radioluscent lesion | 2.0×3.0 | 2.0×3.0 |
| 27 | This case | 41/F | Right condylar neck | Swelling, pain, conductive hearing loss, restricted mouth opening | Well-defined radiopaque mass with thin radioluscent rim | 7.0×6.8×6.4 (CT scan) | 7.0×6.5 |
(M: male, F: female, NS: not specified, NA: data not available, CT: computed tomography)
Some cases were adapted from the articles of Singh and Solomon (J Dent Sci 2012. doi: 10.1016/j.jds.2012.10.002. [Epub ahead of print])4 and An et al. (Oral Surg Oral Med Oral Pathol Oral Radiol 2013;116:e134-40)5.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download