Abstract
Objectives
To compare the efficacy of intravenous ondansetron (4 mg, 2 mL) and granisetron (2 mg, 2 mL) for preventing postoperative nausea and vomiting (PONV) in patients during oral and maxillofacial surgical procedures under general anesthesia.
Materials and Methods
A prospective, randomized, and double blind clinical study was carried out with 60 patients undergoing oral and maxillofacial surgical procedures under general anesthesia. Patients were divided into two groups of 30 individuals each. Approximately two minutes before induction of general anesthesia, each patient received either 4 mg (2 mL) ondansetron or 2 mg (2 mL) granisetron intravenously in a double blind manner. Balanced anesthetic technique was used for all patients. Patients were assessed for episodes of nausea, retching, vomiting, and the need for rescue antiemetic at intervals of 0-2, 3, 6, 12, and 24 hours after surgery. Incidence of complete response and adverse effects were assessed at 24 hours postoperatively. Data was tabulated and subjected to statistical analysis using the chi-square test, unpaired t-test, or the Mann-Whitney U-test as appropriate. A P-value less than 0.05 was considered statistically significant.
Results
There was no statistically significant difference between the two groups for incidence of PONV or the need for rescue antiemetic. Both study drugs were well tolerated with minimum adverse effects; the most common adverse effect was headache. The overall incidence of complete response in the granisetron group (86.7%) was significantly higher than the ondansetron group (60.0%).
Conclusion
Granisetron at an intravenous dose of 2 mg was found to be safe, well tolerated, and more effective by increasing the incidence of complete response compared to 4 mg intravenous ondansetron when used for antiemetic prophylaxis in maxillofacial surgery patients receiving general anesthesia. Benefits of granisetron include high receptor specificity and high potency, which make it a valuable alternative to ondansetron.
Oral and maxillofacial surgical procedures under general anesthesia have a relatively common complication of postoperative nausea and vomiting (PONV). Postoperative vomiting may predispose patients to increased pain, bleeding, dehydration, electrolyte imbalance, and delayed wound healing1. Thus, it is prudent to minimize or prevent vomiting in such patients.
There are a number of drugs that are used to manage PONV including antihistaminics, phenothiazine derivatives, anticholinergics, and dopamine receptor antagonists. However, these drugs cause unwanted side effects such as sedation, dysphoria, extrapyramidal symptoms, dry mouth, restlessness, and tachycardia. The recently introduced 5-hydroxytryptamine (5-HT) receptor antagonists are devoid of such side effects and are highly effective in the prevention durand treatment of PONV. The drug commonly used to treat PONV is ondansetron (4 mg) via an intravenous route2.
The prophylactic efficacy of ondansetron in preventing PONV has already been established in various surgical procedures requiring general anesthesia34567. However, with regard to oral and maxillofacial surgical procedures under general anesthesia, some studies have shown ineffectiveness of 4 mg ondansetron prophylaxis, whereas another study has shown that ondansetron is more effective than metoclopramide189. Therefore, the efficacy of ondansetron in preventing PONV has not been clearly established in patients undergoing oral and maxillofacial surgical procedures under general anesthesia.
Granisetron is another recently introduced 5-HT receptor antagonist which has good potency and a longer duration of action against emesis. The optimal dose of granisetron in preventing PONV is 2 mg via an intravenous route. We did not discover any literature comparing any other highly effective prophylactic drug with ondansetron for prevention of PONV in maxillofacial surgical patients under general anesthesia.
Therefore, this study was undertaken to compare the efficacy of intravenous ondansetron 4 mg (2 mL) and granisetron 2 mg (2 mL) in preventing PONV in patients undergoing oral and maxillofacial surgical procedures under general anesthesia.
This prospective and randomized clinical study was completed at Bapuji Dental College and Hospital (Davangere, India) from October 2011 to October 2012 and was approved by the Institutional Ethics Committee of Bapuji Dental College and Hospital. Sixty patients of either sex were allocated into two equal groups in a double blind and randomized manner. Individuals between the ages of 18 to 70 years, weighing more than 40 kg, having American Society of Anesthesiologists (ASA) I-II category, and those scheduled for oral and maxillofacial surgery under general anesthesia were included in the study. Patients were excluded if they were unable or unwilling to give informed consent, had documented hypersensitivity to any of the study drugs, had a history of motion sickness or previous PONV, had taken antiemetic drugs within 24 hours before surgery, had a history of neurological or renal diseases, were medically compromised and not considered fit for surgery under general anesthesia, or had a past history of adverse reactions to the study drugs.
One day prior to surgery, all patients received a pre-anesthetic evaluation by the anesthetist. The night before surgery, all patients were given oral diazepam (5 mg) and ranitidine (150 mg) and kept nil per os (nothing by mouth) after 10:00 p.m. followed by oral diazepam (5 mg) and ranitidine (150 mg) at 6:00 a.m. on the day of surgery as per the anesthetist's order. Upon arrival in the operation theatre, patients were connected to the intravenous line and intravenous fluids were started; monitors for electrocardiogram, pulse-oximeter, and noninvasive blood pressure (NIBP) were also connected and set at monitoring mode. Patients were premedicated with an injection of midazolam (0.05 mg/kg) and pentazocine (0.5 mg/kg).
Patients were randomly allocated to the two study drug groups (A and B) and in a double blind manner; the anesthetist administered one of the groups intravenously followed by induction using injections of thiopentone sodium (5 mg/kg) and glycopyrrolate (0.2 mg) two minutes later. All patients received a suxamethonium (2 mg/kg) injection for relaxation and then were intubated with an appropriate size endotracheal tube with a throat pack. Patients were maintained on controlled ventilation with oxygen, nitrous oxide, and halothane 0.05% to 1%, and muscle relaxation was maintained with an injection of vecuronium (0.05 mg/kg). Routine prophylaxis was performed with intravenous injection of amoxicillin (1 g) and intravenous dexamethasone (8 mg) was administered to counteract swelling during the postoperative period. Once the surgical procedure was completed, halothane and nitrous oxide were discontinued and the patient was ventilated with 100% oxygen. The throat pack was then removed, oral suctioning was completed, and an appropriate size nasogastric tube was inserted. Patients were reversed with injections of neostigmine (0.05 mg/kg) and glycopyrrolate. They were extubated and a nasopharyngeal airway was established, then patients were transferred to a post anaesthetic care unit (PACU). In the PACU, patients were supplemented with oxygen. Monitors including a pulse-oximeter, electrocardiogram, and NIBP were attached and placed in monitoring mode. Once adequate recovery was achieved, the patients were transferred to the department wards.
Postoperatively, all patients were assessed at the PACU and department wards for episodes of nausea, retching, vomiting, and the need for rescue antiemetic at intervals of 0-2, 3, 6, 12, and 24 hours. Episodes of PONV were identified by spontaneous complaints from patients or by direct questioning. The patients were observed for 24 hours postoperatively for incidence of complete response and adverse effects.
"Complete response" was defined as the absence of nausea, retching, or vomiting and no need for rescue antiemetic during the 24 hour observation period. Rescue antiemetic in the form of an intravenous injection of metoclopramide (10 mg, PERINORM; IPCA Labs, Mumbai, India) was given in the event of one or more episodes of vomiting depending on the observer's discretion.
These postoperative assessments were recorded on a standardized form. Upon completion of the study, un-blinding revealed that group A (n=30) received 2 mL (4 mg) ondansetron (NEOMIT; Neon Laboratories Ltd., Mumbai, India) and group B (n=30) received 2 mL (2 mg) granisetron (GRANIFORCE; Mankind Pharma, New Delhi, India). The data was subjected to statistical analysis by a chi-square test, unpaired t-test, or the Mann-Whitney U-test as appropriate (SPSS version 13; SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was considered significant.
All surgical procedures were performed by a single oral and maxillofacial surgeon. The surgical procedures performed in this study included oncologic resections and reconstructions, excision and reconstruction of surgical pathologies, open reduction and internal fixation of panfacial fractures, and bi-jaw orthognathic surgical procedures.(Table 1) The two groups were comparable with regard to the different types of surgical procedures performed. The demographic data of the study population is presented in Table 2. The present study included 20 males and 10 females with a mean age of 38.0±14.9 years in group A, and 23 males and 7 females with a mean age of 31.8±11.1 years in group B. The mean body weight was 59.2±13.1 kg in group A and 56.0±11.8 kg in group B. The mean duration of surgery in groups A and B was 121.8±84.9 minutes and 139.4±80.3 minutes, respectively. The mean duration of anesthesia was 150.4±85.1 minutes in group A and 166.4±85.0 minutes in group B. The two groups contained individuals with comparable demographics.
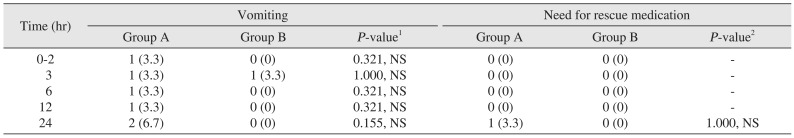
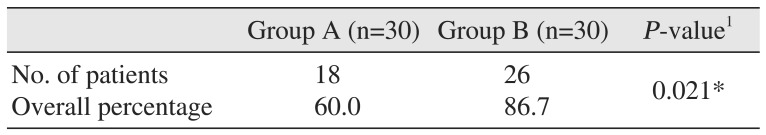
The incidence of nausea was 16.7% (n=5) in group A and 3.3% (n=1) in group B over a 24-hour period (P>0.05).(Table 3) One patient in both groups had an episode of retching over a 24-hour period (P>0.05).(Table 3) Similarly, the results for vomiting when compared between the two groups were not statistically significant (P>0.05).(Table 4) When examining the need for rescue medication, only a single patient in the ondansetron group needed an injection of metoclopramide (10 mg) as compared to no patients in the granisetron group over the 24-hour period (P>0.05).(Table 4) Headache was the only adverse effect occurring more frequently in group A (n=3, 10.0%) as compared to group B (n=1, 3.3%); however, the result was statistically not significant (P>0.05). The incidence of complete response over a 24-hour postoperative period was 60.0% (n=18) in group A as compared to 86.7% (n=26) in group B, which was statistically significant (P=0.021, P<0.05).(Table 5)
In the practice of oral and maxillofacial surgery, PONV is a common problem with approximately 21% to 63% of oral and head and neck surgery patients reporting such symptoms9. When separating postoperative nausea from vomiting, the overall incidence of nausea ranges from 22% to 41%, while that of vomiting ranges from 12% to 33%. PONV is an important factor contributing to patient discomfort and dissatisfaction regarding their office, clinic, or hospital experience. Studies report that avoiding postoperative nausea, vomiting, retching, and gagging on the endotracheal tube are greater concerns among patients than postoperative pain10.
Patients undergoing oral and maxillofacial surgery under general anesthesia are at a moderate to high risk for PONV as most of these surgeries have a long duration with oral/nasal oozing leading to ingestion of blood and involve the use of postoperative opioids10. Such a scenario makes prophylactic anti-emetic treatment an attractive option.
It is well appreciated that a number of factors including various insults, chemotherapeutic agents, radiation, etc. may lead to the release of serotonin from the enterochromaffin of the gastrointestinal tract. Released serotonin may then bind to certain 5-HT receptors and promote nausea/vomiting. The highest concentration of 5-HT receptors is found in the solitary tract nucleus (STN) and the chemoreceptor trigger zone (CTZ) of the central nervous system. It is believed that 5-HT receptor antagonists suppress nausea and vomiting at these STN and CTZ sites. All 5-HT receptor antagonists have the same basic double nitrogen ring backbone within their chemical structure. This may be the chemical site of action of the 5-HT receptor antagonists on serotonin, which is nitrogen based and contains a six and five member ring11. The 5-HT receptor antagonists prevent serotonin from activating and sensitizing the vagal afferent nerves, which causes nausea and vomiting. The 5-HT receptor antagonists ameliorate nausea/vomiting in a number of circumstances and have been utilized as important antiemetics for multiple conditions such as chemotherapy-induced nausea/vomiting, radiation-induced emesis, and PONV12.
The development of selective 5-HT receptor antagonists has dramatically improved the treatment of nausea and vomiting12. Ondansetron is a commonly used 5-HT receptor antagonist to prevent patient nausea and vomiting. Its onset of action is less than 30 minutes after an intravenous injection with a duration of 12 to 24 hours. The mean elimination half-life is four hours in adults, while most paediatric patients below 15 years of age have a shorter plasma half-life of 2.4 hours13. Granisetron is a recently introduced 5-HT receptor antagonist which has good potency and a longer duration of action against emesis. The onset of action following a single intravenous dose of granisetron is within 30 minutes and with a duration of more than 24 hours. Its elimination half-life is 8.95 hours14. Granisetron is a highly selective 5-HT receptor antagonist that has little or no affinity for other receptors, a characteristic that is thought to underlie its favorable side effects and safety profile. Extensive clinical trial data have shown granisetron to be an effective and well-tolerated agent for the treatment of nausea and vomiting in oncology and surgical settings15. The higher control rate with granisetron may be due to its higher specificity and affinity for 5-HT receptors and its longer serum half-life compared to other agents15.
The etiology of PONV is complex and dependent on a variety of factors, including age, obesity, a history of motion sickness and/or previous PONV, menstruation, surgical procedure, anesthetic technique, and postoperative pain16. In our study, the treatment groups were comparable with respect to patient demographics, surgical procedures performed, anesthetics administered (balanced anesthesia), and analgesics used postoperatively. Patients with a history of motion sickness and/or previous PONV, and those who were menstruating were excluded because of a relatively high risk of PONV. Therefore, the difference in a complete response (no PONV, no rescue medication) between the groups can be attributed to the study drug.
One drawback of our study design was the lack of a control group receiving a placebo. Studies have shown that ondansetron and granisetron are better antiemetics for preventing PONV compared to a placebo46717181920. Aspinall and Goodman have suggested that placebo controlled trials may be unethical if active drugs are available, because PONV are common and distressing symptoms against which there is effective treatment16. Therefore, a control group was not included in our study.
The recommended dose of ondansetron for PONV prophylaxis is 4 mg561721. In our study, we selected an intravenous dosage of 4 mg ondansetron based on previous studies by McKenzie et al.4, Honkavaara5, Kovac et al.17, and prophylactic ondansetron-meta analysis by Figueredo and Canosa21. Granisetron (40 µg/kg) is considered an appropriate dosage for preventing postoperative emesis after anesthesia16. The intravenous 2 mg dosage of granisetron was based on the study by Bhattacharya and Banerjee2.
In our study, the drugs were administered two minutes before the induction of anesthesia based on previous studies by Honkavaara5 and Bhattacharya and Banerjee2. Ondansetron and granisetron reached a peak plasma concentration within 30 minutes of intravenous administration22. Hence intravenous administration just before induction provides a sufficient postoperative antiemetic effect.
Postoperative assessment of nausea, retching, and vomiting at 0-2, 3, 6, 12, and 24-hour intervals in both ondansetron and granisetron groups was found to be statistically insignificant (P>0.05). This finding is comparable to the study by Bestas et al.22, who compared the effects of ondansetron and granisetron on PONV in adult patients undergoing laparoscopic cholecystectomy and observed no significant differences in PONV between the active treatment groups.
In our study, patients in both the ondansetron and granisetron groups did not receive rescue antiemetic during the 0-2, 3, 6, and 12-hour intervals. At the 24-hour interval, one patient out of 30 (3.3%) in the ondansetron group received rescue antiemetic in the form of intravenous metoclopramide (10 mg), while no patients in the granisetron group received rescue antiemetic. These results were not statistically significant and comparable to studies reported in the literature22223.
Both drugs were relatively well-tolerated and had minimal adverse effects. In the ondansetron group, three patients (10.0%) complained of headache whereas as one patient (3.3%) reported similar in the granisetron group. These results were comparable to studies by Figueredo and Canosa21 that showed a 7.05% incidence of headache with ondansetron and studies by Fujii et al.16 that showed a 2% to 5% incidence of headache with granisetron. Dizziness, rashes, allergic reactions, and other adverse effects were not reported in the entire study population.
In our study, complete response occurred in 60% of the cases in the ondansetron group, which is comparable to the studies conducted by Naguib et al.23 (65.5%) and Kovac et al.11 (64%). The complete response in the granisetron group occurred in 86.7% cases, which is comparable to the work done by Mikawa et al.19 (83%) and Fujii et al.161820 (85%). This result was statistically significant (P=0.021; P<0.05) and similar to the study by Bhattacharya and Banerjee2. However, the incidence of complete response in our study (complete response=60.0% in ondansetron group and 86.7% in granisetron group) was less than previously reported by Bhattacharya and Banerjee2 (complete response=80% in ondansetron group and 93% in granisetron group). This may be explained by a difference in the type and duration of surgical procedures included in the present study, as tubal ligations were also included in their study.
In conclusion, granisetron at an intravenous dose of 2 mg was found to be safe, well tolerated, and more effective than a 4 mg intravenous ondansetron for antiemetic prophylaxis in maxillofacial surgery patients receiving general anesthesia, and can be employed as routine antiemetic prophylaxis for PONV. The benefits of granisetron, including high receptor specificity and high potency, make it a valuable alternative to ondansetron. The present study has not addressed the issues of economy and surrogate variables such as hospital discharge times, expenses incurred towards treating established PONV, or sequelae of PONV, which can be considered shortcomings of this study. Nevertheless, a study addressing these issues should be carried out in the near future.
References
1. Talesh KT, Motamedi MH, Kahnamouii S. Comparison of ondansetron and metoclopramide antiemetic prophylaxis in maxillofacial surgery patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111:275–277. PMID: 20674417.

2. Bhattacharya D, Banerjee A. Comparison of ondansetron and granisetron for prevention of nausea and vomiting following day care gynaecological laparoscopy. Indian J Anaesth. 2003; 47:279–282.
3. Leeser J, Lip H. Prevention of postoperative nausea and vomiting using ondansetron, a new, selective, 5-HT3 receptor antagonist. Anesth Analg. 1991; 72:751–755. PMID: 1827966.

4. McKenzie R, Kovac A, O'Connor T, Duncalf D, Angel J, Gratz I, et al. Comparison of ondansetron versus placebo to prevent postoperative nausea and vomiting in women undergoing ambulatory gynecologic surgery. Anesthesiology. 1993; 78:21–28. PMID: 8424561.

5. Honkavaara P. Effect of ondansetron on nausea and vomiting after middle ear surgery during general anaesthesia. Br J Anaesth. 1996; 76:316–318. PMID: 8777119.

6. Dershwitz M, Conant JA, Chang Y, Rosow CE, Connors PM. A randomized, double-blind, dose-response study of ondansetron in the prevention of postoperative nausea and vomiting. J Clin Anesth. 1998; 10:314–320. PMID: 9667348.

7. Liberman MA, Howe S, Lane M. Ondansetron versus placebo for prophylaxis of nausea and vomiting in patients undergoing ambulatory laparoscopic cholecystectomy. Am J Surg. 2000; 179:60–62. PMID: 10737581.

8. Rodrigo C, Campbell R, Chow J, Tong A. The effect of a 4-mg preoperative intravenous dose of ondansetron in preventing nausea and vomiting after maxillofacial surgery. J Oral Maxillofac Surg. 1996; 54:1171–1175. PMID: 8859234.

9. Wagley C, Hackett C, Haug RH. The effect of preoperative ondansetron on the incidence of postoperative nausea and vomiting in patients undergoing outpatient dentoalveolar surgery and general anesthesia. J Oral Maxillofac Surg. 1999; 57:1195–1200. PMID: 10513865.

10. Kovac AL. The prophylactic treatment of postoperative nausea and vomiting in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2005; 63:1531–1535. PMID: 16182924.

11. Kovac AL, O'Connor TA, Pearman MH, Kekoler LJ, Edmondson D, Baughman VL, et al. Efficacy of repeat intravenous dosing of ondansetron in controlling postoperative nausea and vomiting: a randomized, double-blind, placebo-controlled multicenter trial. J Clin Anesth. 1999; 11:453–459. PMID: 10526822.

12. Smith HS, Cox LR, Smith EJ. 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann Palliat Med. 2012; 1:115–120. PMID: 25841471.
13. Pasricha PJ. Prokinetic agents, antiemetics, and agents used in irritable bowel syndrome. In : Goodman LS, Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gilman's the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill;2001. p. 1021–1036.
14. Facts and Comparisons (Firm). Drug facts and comparisons 2003. 57th ed. St. Louis: Facts and Comparisons;2003.
15. Aapro M. Granisetron: an update on its clinical use in the management of nausea and vomiting. Oncologist. 2004; 9:673–686. PMID: 15561811.

16. Fujii Y, Saitoh Y, Tanaka H, Toyooka H. Comparison of ramosetron and granisetron for preventing postoperative nausea and vomiting after gynecologic surgery. Anesth Analg. 1999; 89:476–479. PMID: 10439770.

17. Kovac AL, Pearman MH, Khalil SN, Scuderi PE, Joslyn AF, Prillaman BA, et al. S3A-379 Study Group. Ondansetron prevents postoperative emesis in male outpatients. J Clin Anesth. 1996; 8:644–651. PMID: 8982892.

18. Fujii Y, Tanaka H, Toyooka H. Optimal anti-emetic dose of granisetron for preventing postoperative nausea and vomiting. Can J Anaesth. 1994; 41:794–797. PMID: 7954995.

19. Mikawa K, Takao Y, Nishina K, Maekawa N, Obara H. The antiemetic efficacy of prophylactic granisetron in gynecologic surgery. Anesth Analg. 1995; 80:970–974. PMID: 7726441.

20. Fujii Y, Toyooka H, Tanaka H. Granisetron reduces the incidence of nausea and vomiting after middle ear surgery. Br J Anaesth. 1997; 79:539–540. PMID: 9389277.
21. Figueredo ED, Canosa LG. Ondansetron in the prophylaxis of postoperative vomiting: a meta-analysis. J Clin Anesth. 1998; 10:211–221. PMID: 9603591.

22. Bestas A, Onal SA, Bayar MK, Yildirim A, Aygen E. Effects of ondansetron and granisetron on postoperative nausea and vomiting in adult patients undergoing laparoscopic cholecystectomy: a randomized, double-blind, placebo-controlled clinical trial. Curr Ther Res Clin Exp. 2007; 68:303–312. PMID: 24692762.

23. Naguib M, el Bakry AK, Khoshim MH, Channa AB, el Gammal M, el Gammal K, et al. Prophylactic antiemetic therapy with ondansetron, tropisetron, granisetron and metoclopramide in patients undergoing laparoscopic cholecystectomy: a randomized, doubleblind comparison with placebo. Can J Anaesth. 1996; 43:226–231. PMID: 8829860.

Table 1
Classification of surgical procedures between the two groups
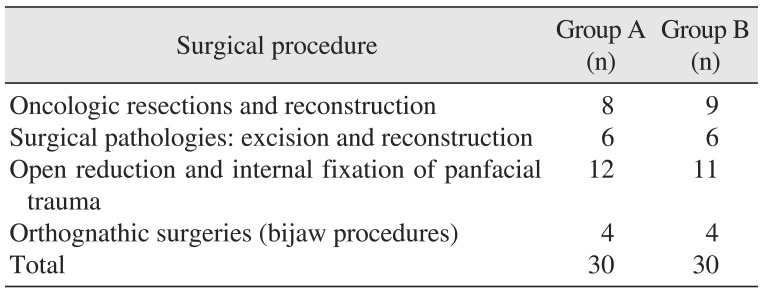
Table 2
Demographic data of the study population
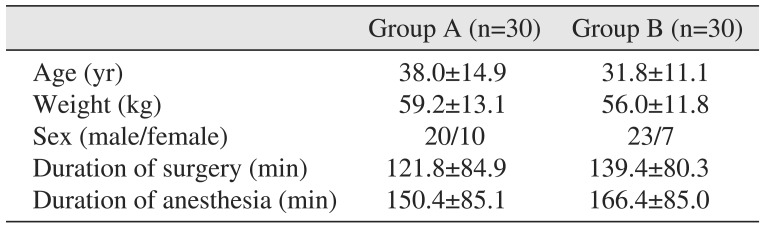
Table 3
Assessment of postoperative nausea and retching
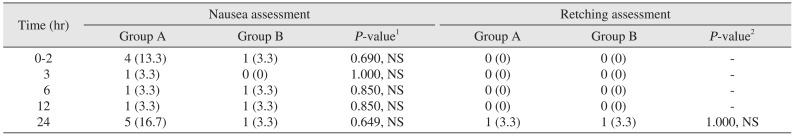
Table 4
Assessment of postoperative vomiting and need for rescue medication




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download