Abstract
Dracunculiasis, otherwise known as guinea worm disease (GWD), is caused by infection with the nematode Dracunculus medinensis. This nematode is transmitted to humans exclusively via contaminated drinking water. The transmitting vectors are Cyclops copepods (water fleas), which are tiny free-swimming crustaceans usually found abundantly in freshwater ponds. Humans can acquire GWD by drinking water that contains vectors infected with guinea worm larvae. This disease is prevalent in some of the most deprived areas of the world, and no vaccine or medicine is currently available. International efforts to eradicate dracunculiasis began in the early 1980s. Most dentists and maxillofacial surgeons have neglected this kind of parasite infection. However, when performing charitable work in developing countries near the tropic lines or other regions where GWD is endemic, it is important to consider GWD in cases of swelling or tumors of unknown origin. This paper reviews the pathogenesis, epidemiology, clinical criteria, diagnostic criteria, treatment, and prevention of dracunculiasis. It also summarizes important factors for maxillofacial surgeons to consider.
Dracunculiasis, also known as guinea worm disease (GWD), is a parasitic infection of humans caused exclusively by the water-borne nematode Dracunculus medinensis. The common name of this disease is derived from its prevalence on the gulf of Guinea. Moreover, this disease has been known to humans since antiquity123. Dracunculiasis was first mentioned in the Turin Papyrus in the fifteenth century BC by the Egyptians and is also described in the Old Testament. Since then, it has also been described by ancient Greek, Roman, Arab, Persian, and Indian physicians45. The "fiery serpents" of the Israelites are presently believed to be guinea worms. Until recently, the people of the Dogon region in Mali referred to GWD as "the disease of the empty granary"345.
Dracunculiasis can cause temporary incapacitation and can sometimes be associated with permanent disability, resulting in fatal damage to the body. It is highly prevalent in economically disadvantaged communities in tropical regions, including Africa and Southern Asia, and has been associated with reduced economic status and low levels of education67. Although rapid and significant progress has been made worldwide regarding the prevention and treatment of GWD, thus reducing the incidence of GWD by more than 99%, some developing countries still have high rates of morbidity and mortality89.
In the field of oral and maxillofacial health care, only a single report10 has described a case of dracunculiasis; this case was from a developing tropical country. Most dentists and maxillofacial surgeons have neglected or are not even familiar with this parasitic infection or its sequelae. However, since the amount of charitable work (i.e., free-of-charge surgery) in developing countries has increased recently, it is important for dentists and maxillofacial surgeons to have accurate knowledge of and to be familiar with the appropriate treatment protocols for this frequent tropical disease. When we found any swelling or tumors of unknown origin in patients in developing countries near the tropic lines, parasite infection (e.g., dracunculiasis) was a tentative clinical diagnosis.
This paper reviews the pathogenesis, epidemiology, clinical criteria, diagnostic criteria, treatment, and prevention of dracunculiasis. It also summarizes important factors for maxillofacial surgeons to consider.
Dracunculiasis is transmitted exclusively to humans via the ingestion of drinking water contaminated with D. medinensis-infected Cyclops, a copepod that is the intermediate host of this parasite. After drinking the water, the Cyclops fleas die and release D. medinensis larvae, which penetrate the human stomach and intestinal wall and then enter the abdominal cavity and retroperitoneal space. These third-stage larvae develop and mature into adults. Male worms die after copulation, whereas females grow to a length of 60 to 100 cm and migrate toward the subcutaneous skin tissue. At approximately 10 to 14 months after ingesting the contaminated water, the female worm induces a painful blister that is predominantly found on the skin of the lower extremities. When the blister ruptures, larvae are released on contact with water by emergence of the adult female worm. These immature first-stage larvae can live in the water for 3 days and can be ingested by Cyclops water fleas to become second-stage larvae. The infectious third-stage larvae develop after about two more weeks, after which the transmission cycle is perpetuated by humans drinking stagnant unfiltered water containing infected Cyclops fleas12345811.(Fig. 1)
No vaccine has yet been developed for the mass treatment of this disease1213. However, considering the life cycle of the guinea worm, various opportunities exist for prevention of GWD transmission at different points. For instance, contaminated sources of drinking water can be avoided, unsafe water can be filtered with cloth and fine-mesh strainers, sources of drinking water can be improved, and adequate measures can be taken to control the vectors of transmission.
Adult D. medinensis parasites mature only in humans over a period of 10 to 14 months, during which the infection manifests no symptoms or outward signs. After this period, a thin white female adult worm, which is up to 60 to 100 cm long and 1.5 to 2.0 mm thick, emerges slowly and painfully through a ruptured blister on the skin561415. Female worms inhabit human subcutaneous tissue and can emerge from any skin surface on the body, including the face, abdomen, arm, and lower extremities16 (Fig. 2); however, most female worms emerge from the leg, ankle, or foot. A dozen or more worms have even emerged simultaneously from a single patient.
Most of the interior body of the female worm is occupied by the uterus, which contains thousands of first-stage larvae561415. A blister is formed on the human skin around the anterior end of the worm; this blister ruptures when it is exposed to water. The female worm protrudes its anterior end and discharges first-stage larvae (650×20 µm) into the water and continues to protrude and release larvae into water for the following 2 to 6 weeks, after which the female worm dies. First-stage larvae can infect water fleas in freshwater by being swallowed. Within the water fleas, second-stage larvae develop and penetrate the gut wall of the water flea. After approximately 2 weeks, the larvae molt twice to become thirdstage larvae (450×14 µm). The vectors that harbor infectious third-stage larvae become sluggish and sink to the bottom of the water. Many species of Cyclops copepods have been found to be naturally infected with D. medinensis species in various regions of the world1718.
Cyclops copepods ingested via drinking water are dissolved by the gastric juice of the stomach, after which the infectious larvae penetrate the stomach or intestine of the human host. After a period of residence in the abdominal cavity, the larvae migrate into the connective tissues, where they develop into mature worms. Mating occurs about 3 months after the initial infection; the males (1-4 cm×0.4 mm) are much smaller than the females and die after mating. Female worms move through the connective tissues and usually reach the lower extremities by about 10 to 14 months postinfection.(Fig. 1)
Although parasites resembling D. medinensis have been observed in dogs and other animals, no other host or environmental reservoir of D. medinensis has been found outside of human beings. The immature first-stage larvae survive only a few days outside of the human body and must be ingested by a water flea for the life cycle to continue. For this reason, even though water fleas are ubiquitous in stagnant surface sources of freshwater, GWD is vulnerable to complete eradication. However, more recently the presence of other hosts (i.e., dogs) was proposed as a possible reason for why GWD has been difficult to eradicate19.
Early in the twentieth century, dracunculiasis was widely prevalent in many Asian and African countries, including southern parts of the Soviet Union, India, Persia, and Saudi Arabia, in addition to much of North, East, and West Africa. The global guinea worm eradication program20 was conceived and initiated at the Centers for Disease Control and Prevention21 in Atlanta, Georgia in October 1980. This program was originally intended to be an adjunct to the International Drinking Water Supply and Sanitation Decade (IDWSSD), one of whose goals was to provide safe drinking water to all who did not yet have it. The Carter Center22 initiated another global campaign in 1986, since an estimated 3.5 million people were being infected annually in three Asian and 17 African countries23. By engaging two prominent leaders of African countries, Mali and Nigeria, in the prevention campaign, a dramatic decline of dracunculiasis cases was reported in 20002324.
By the end of 2008, dracunculiasis had been completely eliminated from Asia, the number of endemic countries had been reduced from 20 to 6, and the number of endemic villages had fallen from over 23,000 in 1991 to 1,025. Moreover, the number of cases reported had been reduced from an estimated 3.5 million in 1986 to 4,619 in 2008920. In 2004, the 12 remaining endemic countries pledged to eradicate the disease by 2009. Eight of these 12 countries claimed to have interrupted transmission by the end of 2009, bringing the total number of countries that had interrupted transmission to 16. The number of cases has presently declined to 3,190, which is a 99% reduction since the start of the global campaign. An International Commission for the Certification of Dracunculiasis Eradication20 reviewed data submitted by a number of countries and the reports of teams sent to verify the surveillance status in selected countries. The World Health Organization (WHO) officially certified the absence of dracunculiasis from 180 countries, with 21 countries remaining to be certified.
As of 2012, a total of 14 countries remain to be certified15. These countries include four endemic countries (Chad, Ethiopia, Mali, and South Sudan), six previously endemic countries in the pre-certification stage (Côte d'Ivoire, Ghana, Kenya, Niger, Nigeria, and Sudan), and four countries with no known history of dracunculiasis pending verification (Angola, the Democratic Republic of the Congo, Somalia, and South Africa).(Fig. 3) Dracunculiasis is now confined to a few countries in Africa—Mali, Niger, Ghana, Nigeria, Sudan, and South Sudan—with more than 90% of all cases reported in South Sudan15.(Fig. 4)
Dracunculiasis emerges seasonally, precisely during the time of year of peak agricultural work, when villagers need to harvest or plant their crops2526. In the driest areas such as the Sahel region just below the Sahara desert, the "Guinea worm season" coincides with the brief rainy season, presumably because the sources of surface water are most abundant during this time, leading to high levels of parasite transmission. On the other hand, in moister climates near the Atlantic coast in West Africa, the dry season is the optimal period for the disease. In the moister climates, the dry season is when the stagnant water sources are shrinking and most contaminated, while the abundance of water during the rainy season causes the rivers to flow and dilute any contamination that may be present. Dracunculiasis has a negative impact on human health and also on other societal factors, such as agricultural productivity and school attendance2526.
There are usually no symptoms during the first 10 to 14 months of parasite maturation. The first sign is normally a burning sensation and pruritus that patients experience before the worm penetrates the skin. After these sensations, the dermis of the penetrated area becomes elevated and a blister develops. Patients often place the affected area in water to relieve this pain and discomfort. Adult female worms are most frequently located in the lower extremities, including the foot or lower leg, but can also appear on the upper extremities, breasts, head, back, scrotum, or anywhere else on the bodily surface. When worms emerge close to joints, they can cause arthritis. They can also cause foot and leg joints to become stiff and painful due to inflammatory responses or worm calcification578.
Secondary infection of ulcers can lead to bacterial cellulitis; moreover, these ulcers can contain lesions that can act as points of entry for tetanus spores. Inflammation makes it difficult to extract adult worms from ulcers before the uterus of the worm has completely released its larvae. If no secondary infection is present, guinea worm ulcers can be healed spontaneously after the empty worm has been removed. However, if any part of the adult worm remains after breakage, the remainder of the worm withdraws into the host tissue and causes a severe inflammatory reaction that can result in a larger ulcer and consequent extensive fibrosis. Only a single worm usually appears in each patient per year; however, in rare instances many worms (more than 20) have emerged from a single individual. Some of these female worms die before they emerge through the skin, which is problematic because these dead or ruptured worms can cause subcutaneous abscesses. In other rare instances, worms can migrate into vital organs, where they cause serious problems. Many people in endemic areas have antibodies against the parasite because of current or recent infections. Infections do not confer immunity and thus most people in endemic areas become reinfected year after year.
Unfortunately, guinea worm infections cannot be diagnosed in the prepatent period (i.e., the first 8-10 months of infection). However, sometimes adult female worms are visible or palpable under the skin shortly before they emerge. Clinical diagnosis is made by examining the guinea worm ulcer and observing the female worm protruding from the blister. The distinctive appearance of the blister, which is accompanied by local itching and burning pain, makes diagnosis easy. Active larvae can also be obtained by immersing the protruding adult female in a small tube or container with water. The first stage larvae can be observed under a microscope and are easily identified by their characteristic pointed tails. Serologic inspection is usually of no practical use in diagnosis. Patients often have eosinophilia; dead calcified worms are easily seen in radiographs, whereas live worms are not visible5789.
Although dracunculiasis is not currently treatable, infection transmission can fortunately be prevented in several ways. Specifically, transmission can be prevented by protecting drinking water from contamination by infected humans and by protecting humans from drinking contaminated water. The most basic intervention is to teach people in endemic areas to avoid entering sources of drinking water when a worm is emerging or about to emerge from their body and to always filter any water they drink from an open pond or other stagnant source through a finely woven cloth27. Another effective intervention is to treat potentially contaminated ponds with larvicide. At the recommended concentration, the recommended larvicide is colorless, odorless, tasteless, and harmless to humans, fish, and plants, but lethal to Copepods. Unfortunately, this is the slowest and most expensive of the available preventive measures28.
Complicating prevention efforts, it is also difficult to convince conservative villagers that this disease was caused by water they drank a year ago, as opposed to a strongly held traditional belief they may have regarding its cause. This can be done, but it is not easy. To prevent contamination of water with guinea worm larvae, health education also emphasizes that people with guinea worm ulcers should have their wounds dressed and should not place infected limbs in water that is used for drinking. Focusing on effective sanitation and hygiene, in addition to reward programs for guinea worm eradication posters, are especially recommended in low-income communities.(Fig. 5)
Recently, more systematic prevention efforts targeting specific population groups252728, such as school age children, preschool children, and pregnant women have been introduced. These efforts have included school-based and maternal care programs, and were also cited as key Millennium Development Goals1415.
There are no specific treatments or cures for dracunculiasis. Treatment consists of providing analgesics to relieve pain and antibiotics to mitigate secondary infections, and to extract the guinea worm surgically. A vaccine has not yet been developed.
After penetration from the skin, an ancient practice consisting of careful winding of the emerging worm around a small stick ("winding of the worm") has traditionally been the best way for extracting the worm. The protruding portion of the female worm must be attached to a small stick that is twisted carefully for several days until the worm is completely removed16.(Fig. 6) Since only a few centimeters of a worm can usually be pulled out per day in this fashion, the painful incapacitation associated with this infection normally lasts up to 1 to 2 months or more. Care should be taken not to break the worm. This traditional technique has been effective in many rural regions without advanced medical facilities. However, if a worm is broken while being coaxed to emerge, the remainder of the worm retracts into the body and spills larvae into the tissues, causing severe inflammation and abscess formation. This complication is very painful and requires incisions and drainage to be performed by medically trained personnel at a clinic facility.
The site of worm emergence often acquires secondary infection with various bacteria, which increases the local inflammation and pain that are the hallmarks of this disease. Infection with tetanus bacilli at the site of the ulcer, which forms at the base of the ruptured blister caused by the emerging worm, is the most dangerous secondary complication of dracunculiasis. In this case, administration of antibiotics and adequate cleaning and dressing of the ulcer are important for reducing secondary infections. In addition, tetanus vaccination is recommended. In about one percent of all cases, a worm that emerges in or near a major joint such as the elbow or knee can cause the joint to permanently freeze, with consequences very similar to those of paralytic poliomyelitis. Since infections do not confer immunity, people can be reinfected year after year.
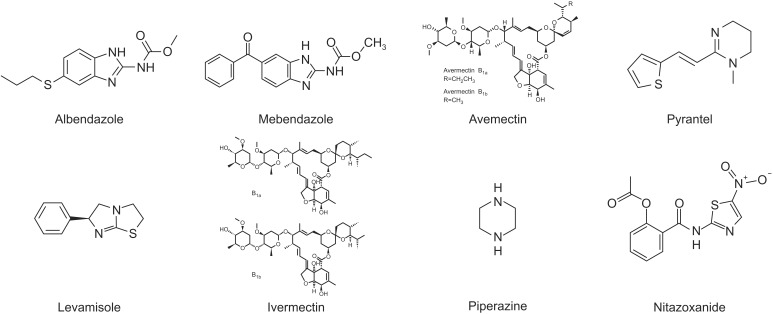
A number of single-dose, orally administered drugs are available for the treatment and control of soil-transmitted helminth (STH) infections. Most of these drugs are broadspectrum benzimidazole anthelmintics29 that were developed in the 1970s. These drugs revolutionized the ability of communities to control geohelminths. Five drugs are currently listed on the WHO's model list of essential medicines needed in a basic health system for the medical treatment of dracunculiasis. These drugs are albendazole, mebendazole, pyrantel, levamisole, and ivermectin.(Fig. 7) Each of these drugs is recommended by the WHO for use in large-scale control program5192930.
Albendazole is a broad-spectrum antihelminthic benzimidazole used most widely for the treatment of a variety of parasitic worm infestations and for community control of multiple STH infections. It can be used to treat giardiasis, trichuriasis, filariasis, neurocysticercosis, hydatid disease, pinworm disease, and ascariasis, among others. Albendazole is taken orally and is poorly absorbed by the gastrointestinal tract. However, it is rapidly and extensively metabolized by the liver to sulphoxide and sulphone metabolites2930. Sulphoxide metabolites are active antihelminthic compounds that are believed to be responsible for most of the in vivo effects of albendazole. These compounds bind to intracellular tubulin, impairing absorption of essential nutrients by the parasite. Common side effects include nausea, abdominal pains, and headaches. Potentially serious side effects include bone marrow suppression; the risk of this effect is higher in patients with liver problems and pregnant women.
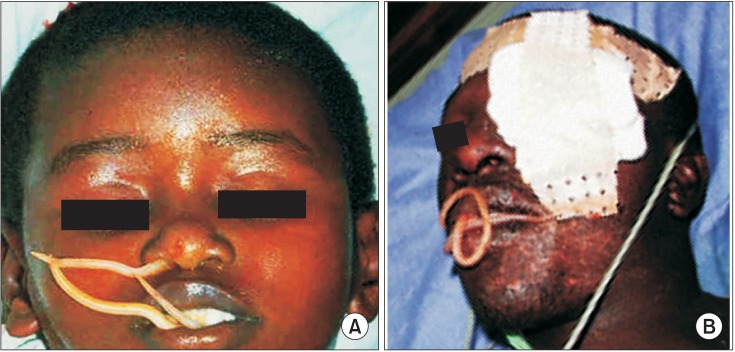
Mebendazole is another well-known broad-spectrum benzimidazole-type antihelminthic agent with activity against the adult worms and especially against the larval stages. This oral drug is effective for treating ascariasis, pinworm disease, hookworm infections, hydatid disease, giardia, and GWD. Mebendazole binds to tubulin in nematodes and prevents the formation of microtubules, thereby selectively inhibiting cell division and glucose uptake in the nematodes. In turn, the helminths use more glycogen, thus depriving the worms of their main source of energy. The adverse effects of mebendazole are mild and transient and include symptoms such as gastrointestinal discomfort, headache, and well-tolerated dizziness2930. Additional common side effects include headache, vomiting, and tinnitus. If used at large doses, mebendazole may cause bone marrow suppression. It is currently unclear whether it is safe to use mebendazole in pregnancy2. In rare instances, mebendazole stimulates Ascaris worms to emerge from the mouth and nostrils, which is an unexpected consequence about which patients should be forewarned16.(Fig. 8)
Abamectin, otherwise known as avemectin, is a natural product of the soil bacterium Streptomyces avermitilis that is fermented to insecticidal and antihelminthic compounds2930. Abamectin is a mixture of two avermectins and consists of more than 80% avermectin B1a (R=CH2CH3) and less than 20% avermectin B1b (R=CH3). These two components—B1a and B1b—have similar toxicological properties and have been used as insecticides, acaricides, and nematicides. Abamectin is effective for controlling insects and mite pests that infect a range of agronomic, fruit, vegetable, and ornamental crops. Moreover, abamectin has also been used to control fire ants and as a veterinary antihelminthic. Unlike other classes of benzimidazoles, resistance against abamectin is rare.
Outside of the benzimidazole derivatives, pyrantel is an antinematodal thiophene and a nicotinic receptor agonist that is also used against the parasites. Like levamisole and the related pyrimidine morantel, it binds to parasite acetylcholine receptors and acts on the neuromuscular system of the worms2930. Spastic muscle paralysis results from prolonged activation of the excitatory nicotinic acetylcholine receptors on the body wall muscle. Pyrantel was often prescribed by veterinarians to treat and prevent the occurrence of intestinal parasites in small animal pets; however, this drug is poorly absorbed by the gastrointestinal tract. Specifically, less than 15% is excreted in the urine in its original form or as metabolites, whereas 70% is excreted in its original form in feces. Common adverse effects include mild and transient gastrointestinal discomfort, headache, dizziness, drowsiness, insomnia, and skin rash. It has not been established whether it is safe to use during pregnancy. Pyrantel and piperazine are antagonistic and should not be administered concurrently.
Levamisole, also known as ergamisol, has a similar mode of action as pyrantel and causes spastic paralysis followed by passive elimination of parasites2930. This drug is rapidly absorbed by the gastrointestinal tract, achieves peak plasma levels within 2 hours, and is eliminated within 3 days. Since much of the absorbed drug is metabolized in the liver, levamisole has also been studied as a chemotherapeutic agent. Levamisole is a synthetic imidazothiazole derivative. Common adverse effects include abdominal pain, nausea, vomiting, dizziness, and headache; the most serious side effect of levamisole is low white blood cells, which makes patients highly vulnerable to infection.
Ivermectin is classified in the avermectin family. This drug causes the cell wall of invertebrates to become more permeable, which results in their death. Ivermectin is broadly effective against many types of parasites, including the causative agents of onchocerciasis, lymphatic filariasis, head lice, scabies, river blindness, and strongyloidiasis2930. In addition, ivermectin is on the WHO's List of Essential Medicines, which contains the most important medications needed in a basic health system31. Although ivermectin has therapeutic properties against Ascaris lumbricoides, it shows only limited effectiveness against Trichuris trichiura and hookworm. Ivermectin can be used topically or orally; common side effects include red eyes, dry skin, and burning skin.
Piperazine is a broad class organic compound with a core piperazine functional group that consists of a six-membered ring containing two nitrogen atoms at opposite positions in the ring.(Fig. 7) Piperazine has many important pharmacological properties and takes the form of small alkaline deliquescent crystals that have a saline taste. This drug is used to treat A. lumbricoides and Enterobius vermicularis infections, especially in the presence of intestinal or biliary obstruction2930.
Nitazoxanide is a broad-spectrum antiparasitic and antiviral drug that is used to treat various helminthic, protozoan, and viral infections. It is the prototype member of the thiazolides, a class of drugs that are synthetic nitrothiazolyl-salicylamide derivatives with antiparasitic and antiviral activity. Tizoxanide, an active nitazoxanide metabolite found in humans, is also a thiazolide antiparasitic drug. The associated adverse effects are generally mild and transient and include abdominal pain, nausea, vomiting, and diarrhea.
In addition to these drugs, a number of new candidates are in development for human use. The first of these, tribendimindine, was initially synthesized in China in the 1980s. Early trials in humans demonstrated that single-dose tribendimidine is effective against all STH species32. However, before tribendimidine can be made available outside China, additional preclinical and clinical studies are required. Additional candidates believed to have promising antihelminthic properties are PF1022A (and its derivative emodepside) and monepantel32.
Global health officials have classified dracunculiasis as one of the helminthic neglected tropical disease (NTDs), which also include schistosomiasis, food-borne trematodes, filariases, STHs, Echinococcosis, and other cestode infections. Because of its ease of transmission and the severity of its related complications, dracunculiasis was the first parasitic NTD slated internationally to be eradicated. Many federal, private, and international agencies are helping the countries that still have local guinea worm cases to eradicate this disease.
Maxillofacial surgeons, who have the responsibility of managing their patient's general health, should be able to accurately and effectively diagnose and manage this human parasitic disease. In recent times, many opportunities for charitable work in tropical developing countries have arisen. Thus, when maxillofacial surgeons encounter facial swelling of unknown origin or patients with cervical tumors, GWD should be considered in the preoperative clinical diagnosis. Obtaining a detailed patient history and surveying the patient's living environment are highly important for accurate diagnosis. In addition, postoperative confirmation that the mass has been removed or that the lesions have decreased should be viewed not only in the context of the removal of the worm, but also in the context of potential secondary infections of dracunculiasis.
Notes
References
1. Ruiz-Tiben E, Hopkins DR. Helminthic diseases: dracunculiasis. In : Heggenhougen K, Quah SR, editors. International encyclopedia of public health. Oxford: Elsevier;2008. p. 294–311.
2. Hunter JM. An introduction to guinea worm on the eve of its departure: dracunculiasis transmission, health effects, ecology and control. Soc Sci Med. 1996; 43:1399–1425. PMID: 8913009.

3. Fenwick A. Waterborne infectious diseases--could they be consigned to history? Science. 2006; 313:1077–1081. PMID: 16931751.

4. Kappagoda S, Ioannidis JP. Neglected tropical diseases: survey and geometry of randomised evidence. BMJ. 2012; 345:e6512. PMID: 23089149.

5. Muller R. Guinea worm disease--the final chapter? Trends Parasitol. 2005; 21:521–524. PMID: 16150643.
6. Behbehani K. Candidate parasitic diseases. Bull World Health Organ. 1998; 76(Suppl 2):64–67. PMID: 10063677.
7. Hopkins DR, Ruiz-Tiben E, Downs P, Withers PC Jr, Roy S. Dracunculiasis eradication: neglected no longer. Am J Trop Med Hyg. 2008; 79:474–479. PMID: 18840732.

8. Hopkins DR, Ruiz-Tiben E. Strategies for dracunculiasis eradication. Bull World Health Organ. 1991; 69:533–540. PMID: 1835673.
9. Dracunculiasis eradication--global surveillance summary, 2008. Wkly Epidemiol Rec. 2009; 84:162–171. PMID: 19425252.
10. Poli F. Differential diagnosis of facial acne on black skin. Int J Dermatol. 2012; 51(Suppl 1):24–26. PMID: 23210948.

11. Brandt FH, Eberhard ML. Distribution, behavior, and course of patency of Dracunculus insignis in experimentally infected ferrets. J Parasitol. 1990; 76:515–518. PMID: 2143224.

12. World Health Organization (WHO). Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. Geneva: WHO;2013.
13. Ruiz-Tiben E, Hopkins DR. Dracunculiasis (Guinea worm disease) eradication. Adv Parasitol. 2006; 61:275–309. PMID: 16735167.

14. Al-Awadi AR, Al-Kuhlani A, Breman JG, Doumbo O, Eberhard ML, Guiguemde RT, et al. Guinea worm (Dracunculiasis) eradication: update on progress and endgame challenges. Trans R Soc Trop Med Hyg. 2014; 108:249–251. PMID: 24699360.

15. Callahan K, Bolton B, Hopkins DR, Ruiz-Tiben E, Withers PC, Meagley K. Contributions of the Guinea worm disease eradication campaign toward achievement of the Millennium Development Goals. PLoS Negl Trop Dis. 2013; 7:e2160. PMID: 23738022.

16. Google image search: "ascaris worm" [Internet]. cited 2016 Mar 24. Available from: https://www.google.com.gh/search?q=ascaris+worm&biw=1192&bih=933&source=lnms&tbm=isch&sa=X&sqi=2&ved=0ahUKEwjLgOmqpYbLAhVL7RQKHbSgD9UQ_AUIBigB#imgrc=jt4vMsn_cowbnM%3A.
17. Watts SJ. Dracunculiasis in Africa in 1986: its geographic extent, incidence, and at-risk population. Am J Trop Med Hyg. 1987; 37:119–125. PMID: 2955710.

18. Schneider MC, Aguilera XP, Barbosa da Silva Junior J, Ault SK, Najera P, Martinez J, et al. Elimination of neglected diseases in latin america and the Caribbean: a mapping of selected diseases. PLoS Negl Trop Dis. 2011; 5:e964. PMID: 21358810.

19. Callaway E. Dogs thwart effort to eradicate Guinea worm. Nature. 2016; 529:10–11. PMID: 26738575.

20. Dracunculiasis eradication. Wkly Epidemiol Rec. 2004; 79:234–235. PMID: 15227956.
21. Guinea worm wrap-up #192 [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;cited 2009 Sep 30. Available from: http://www.cdc.gov/ncidod/dpd/parasites/dracunculiasis/moreinfo_dracunculiasis.htm#wrap.
22. Edungbola LD, Withers PC Jr, Braide EI, Kale OO, Sadiq LO, Nwobi BC, et al. Mobilization strategy for guinea worm eradication in Nigeria. Am J Trop Med Hyg. 1992; 47:529–538. PMID: 1449193.

23. Morenikeji O, Asiatu A. Progress in dracunculiasis eradication in Oyo state, South-west Nigeria: a case study. Afr Health Sci. 2010; 10:297–301. PMID: 21327143.
24. Miri ES, Hopkins DR, Ruiz-Tiben E, Keana AS, Withers PC Jr, Anagbogu IN, et al. Nigeria's triumph: dracunculiasis eradicated. Am J Trop Med Hyg. 2010; 83:215–225. PMID: 20682859.

25. Hunter JM. Bore holes and the vanishing of guinea worm disease in Ghana's upper region. Soc Sci Med. 1997; 45:71–89. PMID: 9203273.

26. Hopkins DR, Withers PC Jr. Sudan's war and eradication of dracunculiasis. Lancet. 2002; 360(Suppl):S21–S22. PMID: 12504489.

27. Tayeh A, Cairncross S, Maude GH. The impact of health education to promote cloth filters on dracunculiasis prevalence in the northern region, Ghana. Soc Sci Med. 1996; 43:1205–1211. PMID: 8903124.

28. Tayeh A, Cairncross S, Maude GH. Water sources and other determinants of dracunculiasis in the northern region of Ghana. J Helminthol. 1993; 67:213–225. PMID: 8288853.

29. Reddy M, Gill SS, Kalkar SR, Wu W, Anderson PJ, Rochon PA. Oral drug therapy for multiple neglected tropical diseases: a systematic review. JAMA. 2007; 298:1911–1924. PMID: 17954542.
30. Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008; 299:1937–1948. PMID: 18430913.

31. Cupp EW, Sauerbrey M, Richards F. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin(Mectizan(®)) monotherapy. Acta Trop. 2011; 120(Suppl 1):S100–S108. PMID: 20801094.
32. Yuan G, Xu J, Qu T, Wang B, Zhang R, Wei C, et al. Metabolism and disposition of tribendimidine and its metabolites in healthy Chinese volunteers. Drugs R D. 2010; 10:83–90. PMID: 20698716.

Fig. 1
Schematic drawing of the transmission cycle of Dracunculus medinensis. 1) A person drinks well or pond water containing water fleas (Cyclops) that are infected with mature thirdstage larvae. 2) Stomach gastric juices digest the water fleas, thus releasing worm larvae that move to the abdominal tissue, where they grow and mate. 3) Fertilized female worms migrate to various body regions, usually the lower extremities. 4) At 10 to 14 months after migration, the worm begins to emerge through the skin at a painful blister site. 5) Upon contact with water, the emerging worm releases immature first-stage larvae into the water source. 6) Water fleas consume worm larvae that resist digestion. 7) In two weeks, the larvae mature to third-stage infected larvae within the water flea, which can infect humans.
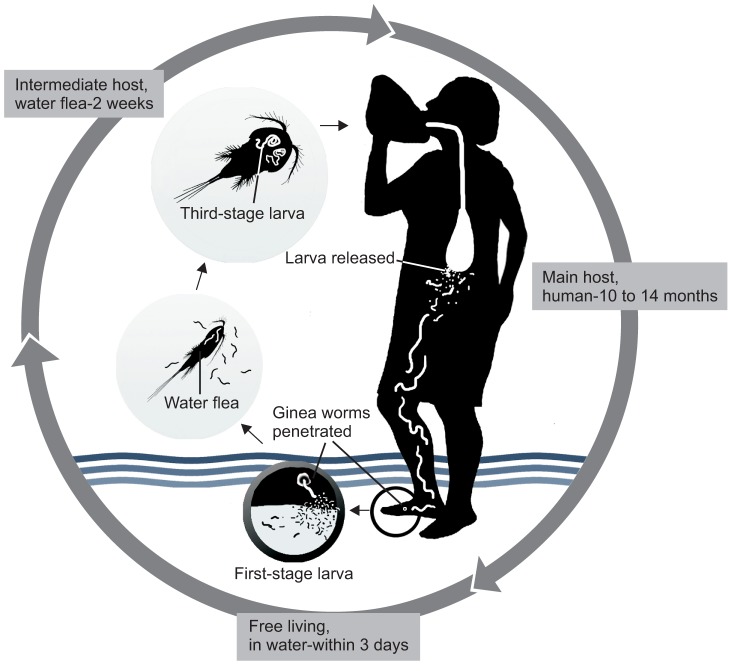
Fig. 2
Skin penetration of the guinea worm through the face (A), ankle (B), arm (C), and abdomen (D). Figures were obtained through Google search16.
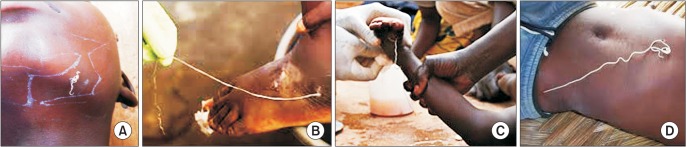
Fig. 3
Endemic status of dracunculiasis in 2012, including precertification and certification countries. Data from the annual reports of World Health Organization (WHO) (Geneva: WHO; 2013)12 and the article of Callahan et al. (PLoS Negl Trop Dis 2013;7:e2160)15.

Fig. 4
Recently reported endemic countries in Africa, including Mali, Niger, Ghana, Nigeria, Sudan, and South Sudan. Data from the annual reports of World Health Organization (WHO) (Geneva: WHO; 2013)12 and the article of Callahan et al. (PLoS Negl Trop Dis 2013;7:e2160)15.
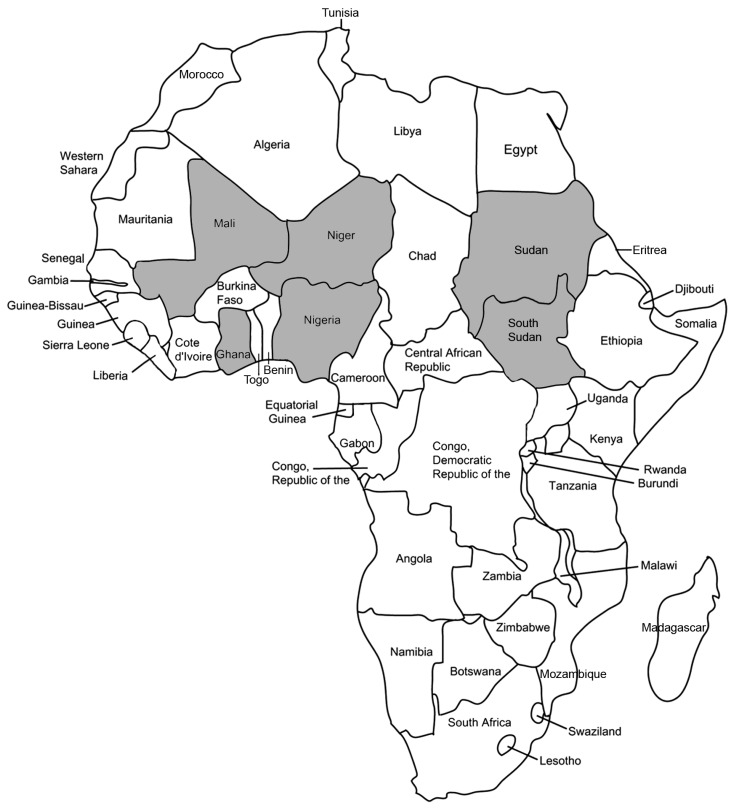
Fig. 5
An example poster of reward programs for guinea worm eradication that was circulated throughout Ghana.

Fig. 6
Winding of a worm around a small stick near the skin penetration wound. Figure was obtained through Google search16
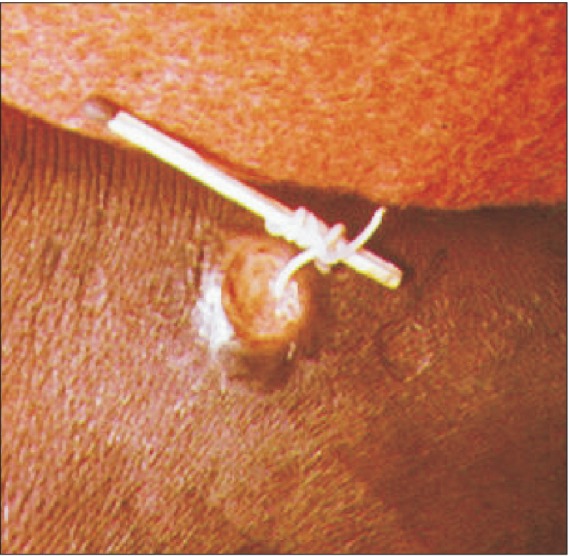
Fig. 8
Ascaris worms emerging from the mouth and nose (A) or mouth (B). Figures were obtained through Google search16.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download