Abstract
Patients with Pierre Robin sequence exhibit varying degrees of airway obstruction and feeding difficulty. In some patients, airway obstruction may be profound, warranting surgical intervention to maintain a patent airway. The purpose of this article is to highlight the advantages of the tongue-lip adhesion procedure for the management of airway obstruction in such patients compared to the currently available options.
Pierre Robin sequence (PRS) is the triad of glossoptosis, micrognathia, and cleft palate1. Varying degrees of airway obstruction are a major problem that may be encountered in these patients. If left untreated, these airway obstructions can lead to acute and chronic hypoxia, apnea, cyanosis, aspirations, respiratory tract infections, feeding difficulty, malnutrition and failure to thrive. The treatment protocol for PRS starts with conservative management, which mostly consists of positional adjustments (prone positioning) and nasopharyngeal airway placement2. Surgical options include tongue-lip adhesion (TLA), glossopexy, tracheostomy and early mandibular lengthening by distraction osteogenesis34. In 1946, Douglas5 described the TLA technique for the management of Pierre Robin patients. TLA is a simple procedure in which the tongue is attached anteriorly to the lower lip. This procedure opens the oropharyngeal airway space as the tongue base is pulled forward.
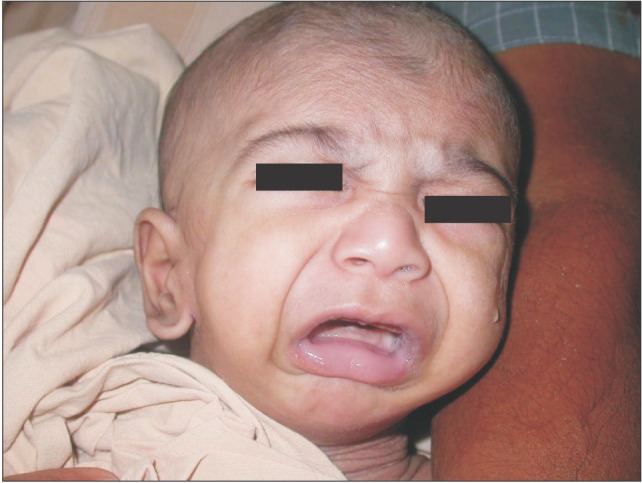
A neonate with PRS (Fig. 1) presented with airway obstruction and respiratory distress after birth. Acute glossoptosis and microretrognathia were managed conservatively with oxygen via nasal cannula and prone positioning during the first few months of life. There was no amelioration of apneic spells despite these measures. Moreover, this child's presentation was complicated by poor weight gain, hence was referred to plastic surgery department and TLA was performed. Following the procedure, patient showed clinical improvements such as weight gain, reduced episodes of respiratory infection and improvement in oxygen saturation. A tonguelip release procedure was performed after one year.
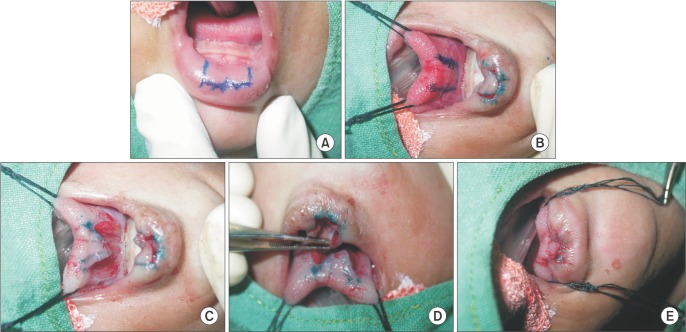
TLA was performed under general anesthesia. Unrestrained tongue protrusion was ensured. The central surface of the tongue was pulled anteriorly to make contact with the lower lip mucosa in order to estimate the necessary raw area. (Fig. 2. A, 2. B) An inferiorly based 2×1-cm flap was raised from the lower lip, and a superiorly based flap of the same size was raised from the ventral surface of the tongue.(Fig. 2. C, 2. D) The raw area was sutured with 5-0 catgut.(Fig. 2. E) Tongue-lip release was performed after one year. After the release procedure, the child was stable and without respiratory distress.(Fig. 3)
Written permission had been obtained from the patient to report and publish the identifying photographs prior itself.
Based on a study by Sher et al.6, in which flexible fiberoptic nasopharyngoscopy was used in 53 infants with PRS, four types of obstruction have been described. In type I obstruction, the most common, obstruction is caused by posterior movement of the tongue against the posterior pharyngeal wall. Type II obstruction is due to posterior and superior displacement of the tongue, promoting contact between the tongue, the velum and the pharyngeal wall in the superior oropharynx. Type III is a pharyngeal obstruction caused by prolapse of the medial pharyngeal wall. Type IV is due to constriction of the pharynx in a circular manner by movement of the tongue and the lateral pharyngeal walls.
Infants with PRS requiring surgical intervention for airway management should be treated with procedures offering the lowest morbidity. Nasaopharyngeal tubes are an unstable mode of therapy, especially for long-term use7. In a recent survey, 91% of pediatric otolaryngology fellowship programs cited tracheostomy as the safest and most reliable surgical method for long-term airway management in infants with PRS3. However, complications related to tracheostomy (tracheal stenosis, granuloma formation, tracheal fistula formation), cannula obstruction and accidental decannulation have been reported in 19% to 49% of patients, and tracheostomy-related mortality ranges from 2% to 8.5%3. Due to the longer associated hospital stays, difficulty in obtaining consent, demand for home care and greater morbidity, especially in newborns, most surgeons avoid tracheostomy.
In tracheostomy, an additional deterrent is that fact that infants who have undergone tracheostomy may demonstrate significant delays in speech production and language development. Unlike with tracheostomy, infants treated with TLA suffer minimal or no adverse effects on their speech development 3. However, TLA is only an appropriate treatment option in infants with type I obstruction without any associated intubaanomalies. In type II, III and IV obstructions, infants do not respond to TLA and tracheostomy may be indicated.
The concept of TLA for obstructive apnoea relief in PRS was first popularised by Douglas in 19463. This procedure involved excising a rectangular area of mucosa on the undersurface of the tongue and extending the area onto the floor of the mouth, alveolus and lower lip. The tongue was brought forward, and the lateral edges of the mucosal incision were sutured to one another, creating broad-based adhesion of the tongue to the lip, alveolus, and floor of the mouth. The procedure was associated with complications like dehiscence, tongue laceration, injury to Wharton's duct and cicatricial ankyloglossia. In 1960, Routledge8 reported a modification that avoided many of these complications. Then, in 1977, Randall 9 described moving the retention suture to the posterior aspect of the tongue, providing better support for the tongue base during healing. In 1992, Argamaso10 later described the use of an internal retention suture looped around the mandible and sutured to the tongue's muscularis propria. He stressed the importance of genioglossus release from its mandibular insertion to allow improved anterior displacement of the tongue11
Serial procedures such as division of TLA (performed after 1 year) and mandibular distraction osteogenesis are performed for cosmetic reasons after facial skeletal maturity has been attained. In the case of a failed TLA, repeat surgery, tracheostomy or early mandibular distraction osteogenesis may be performed.
Mandibular distraction osteogenesis has multiple complications,such as damage to tooth buds, inferior alveolar nerve injury, and unsightly facial scars. Thus, mandibular distraction osteogenesis should be reserved for the small minority of infants who demonstrate persistent apnea despite TLA.
We perform TLA in infants who do not benefit significantly from non-surgical techniques such as prone positioning, supplemental oxygen, oral airway, nasopharyngeal airway, continuous positive airway pressure or endotracheal intubation.
Serial procedures such as division of TLA (performed after 1 year) and mandibular distraction osteogenesis are performed for cosmetic reasons after facial skeletal maturity has been attained. In the case of a failed TLA, repeat surgery, tracheostomy, or early mandibular distraction osteogenesis may be performed.
PRS infants may have associated cleft palates. If cleft palate is present, a repair is required at 1.5 to 2 years of age as was done in this child.
In conclusion, the surgical management of airway obstruction in neonates with Pierre Robin anomaly is dependent on adequate assessment of the site, the mechanism of airway collapse and associated congenital anomalies. TLA can safely be employed with less morbidity in comparison with tracheostomy and mandibular distraction osteogenesis and therefore plays an important role in relieving airway obstruction refractory to positioning and cannulation in neonates.
References
1. Huang F, Lo LJ, Chen YR, Yang JC, Niu CK, Chung MY. Tonguelip adhesion in the management of Pierre Robin sequence with airway obstruction: technique and outcome. Chang Gung Med J. 2005; 28:90–96. PMID: 15880984.
2. Benjamin B, Walker P. Management of airway obstruction in the Pierre Robin sequence. Int J Pediatr Otorhinolaryngol. 1991; 22:29–37. PMID: 1917336.

3. Kirschner RE, Low DW, Randall P, Bartlett SP, McDonald-Mc-Ginn DM, Schultz PJ, et al. Surgical airway management in Pierre Robin sequence: is there a role for tongue-lip adhesion. Cleft Palate Craniofac J. 2003; 40:13–18. PMID: 12498601.

4. Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001; 108:302–311. PMID: 11496167.

5. Douglas B. The treatment of micrognathia associated with obstruction by a plastic procedure. Plast Reconstr Surg (1946). 1946; 1:300–308. PMID: 20278146.

6. Sher AE, Shprintzen RJ, Thorpy MJ. Endoscopic observations of obstructive sleep apnea in children with anomalous upper airways: predictive and therapeutic value. Int J Pediatr Otorhinolaryngol. 1986; 11:135–146. PMID: 3744695.

7. Augarten A, Sagy M, Yahav J, Barzilay Z. Management of upper airway obstruction in the Pierre Robin syndrome. Br J Oral Maxillofac Surg. 1990; 28:105–108. PMID: 2110818.

8. Routledge RT. The Pierre-Robin syndrome: a surgical emergency in the neonatal period. Br J Plast Surg. 1960; 13:204–218. PMID: 13744041.

9. Randall P. The Robin anomalad: micrognathia and glossoptosis with airway obstruction. In : Converse JM, editor. Reconstructive plastic surgery. Philadelphia: WB Saunders;1977. p. 2235–2245.
10. Argamaso RV. Glossopexy for upper airway obstruction in Robin sequence. Cleft Palate Craniofac J. 1992; 29:232–238. PMID: 1591256.

11. Delorme RP, Larocque Y, Caouette-Laberge L. Innovative surgical approach for the Pierre Robin anomalad: subperiosteal release of the floor of the mouth musculature. Plast Reconstr Surg. 1989; 83:960–964. PMID: 2727168.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download