I. Introduction
Oral verruciform xanthoma (OVX) is an uncommon lesion described by Shafer in 1971
1, that appears on oral mucosa as a reactive lesion. The etiopathogenesis of this lesion remains unclear, with reaction response to trauma the most accepted explanation
2. Contrary to skin xanthomas that are associated with metabolic disturbances of lipids, OVXs are not related to any generalized disease
3.
This lesion may occur anywhere in the oral mucosa, but the hard palate is the second most commonly affected site, representing approximately 15% of all cases in a large survey
4.
The treatment for OVX is surgical resection, and recurrence is extremely rare
4. Interestingly, the three recurrence case reports in the English literature occurred in the hard palate
567.
The aim of this paper was to discuss the probable etiopathogenesis of OVX in the hard palate, reinforcing the importance of including this benign lesion in the differential diagnosis of verrucous lesions in this location.
Go to :

II. Case Report
A 43-year-old man presented with a painless lesion in the hard palate, discovered during routine examination two months before. Clinically, a lesion with a verrucous surface and erythematous spots was observed next to the first upper right molar, measuring approximately 5 mm in diameter.(
Fig. 1) The patient was a current smoker. The presumptive diagnosis was squamous cell carcinoma or traumatic ulcer. Excisional biopsy was performed, and the surgical specimen was sent to the Bauru School of Dentistry Oral Pathology Biopsy Service of the University of São Paulo (Bauru, Brazil). Histopathological examination revealed oral mucosa consisting of hyperkeratosis, acanthosis, and elongated rete pegs. Subjacent connective tissue showed numerous foam cells with clear cytoplasm and pyknotic nucleus, negative on periodic acid-Schiff staining.(
Fig. 2. A, 2. B) Immunohistochemical analysis revealed that foam cells were positive for anti-CD68 antibody (
Fig. 2. C), and anti-KI-67 antibody was restricted to the basal layer of the oral epithelium and negative for foam cells.(
Fig. 2. D) Based on clinical and microscopic features, the final diagnosis of OVX was established. After seven months of follow-up, the patient showed no signs of recurrence.(
Fig. 3)
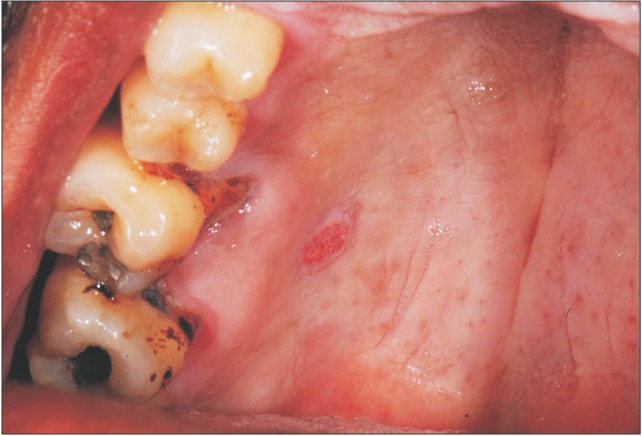 | Fig. 1Clinical appearance of the lesion on the hard palate showing verrucous surface and erythematous spots, measuring approximately 5 mm in diameter.
|
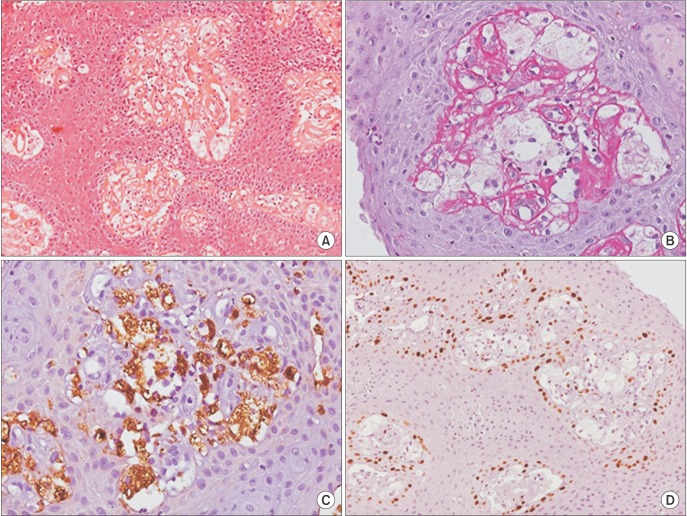 | Fig. 2Histopathological features of the verruciform xanthoma showing hyperkeratosis, acanthosis, elongated rete pegs and numerous foam cells with clear cytoplasm and pyknotic nucleus in the connective tissue (H&E staining, ×200; A), foam cells showing negative for periodic acid-Schiff (PAS staining, ×400; B), foam cells positive for anti-CD68 antibody (anti-CD68 staining, ×400; C), basal layer of the oral epithelium positive to KI-67 and negative for foam cells (anti-KI-67 staining, ×200; D).
|
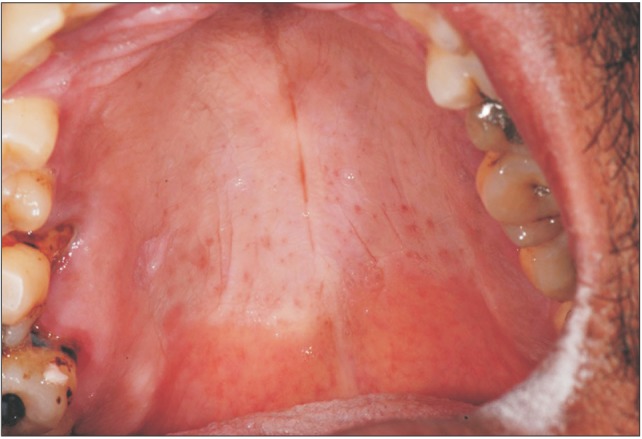 | Fig. 3Seven months of follow-up, no recurrences.
|
Go to :

III. Discussion
OVX is an uncommon lesion that was first described by Shafer in 1971
1. It typically presents as a single lesion, the color of normal oral mucosa with verrucous surface, affecting mostly individuals over 40 years
48. It can be confused clinically with verruca vulgaris, traumatic ulcers, and squamous cell carcinoma.
Histologically, it is characterized by hyperkeratosis, elongated rete pegs, and aggregates of foam cells in the submucosal stroma without epidermal atypia. Foam cells of verruciform xanthoma are of monocyte-macrophage lineage, based on intense cytoplasmic positivity for anti-CD68 and cathepsin B
910.
The hard palate is the second most common area affected by OVX
4. The relationship of this lesion with local trauma can explain why the gums and hard palate are impacted. The trauma induced by cigarettes in this location may also be involved in this lesion. Interestingly, the only three reports of recurrent OVX are in the hard palate
567. This finding reinforces that areas experiencing excessive trauma are more affected by OVX.
OVX usually presents as a small solitary, sessile, or pedunculated lesion with a rough, slightly elevated surface and tends to be asymptomatic. The prevalence of OVX is unknown, although a relative frequency rate of 0.025% to 0.05% has been reported in the literature
211. Such lesions occur most often on the gingiva, followed by hard palate, tongue, buccal mucosa, floor of the mouth, and soft palate
4.
Philipsen et al.
4 showed in a large survey that OVX are common in men below the age of 50 years. In the present case, the patient was a 43-year-old male smoker who presented with a 0.5 cm lesion in the hard palate of two months duration.
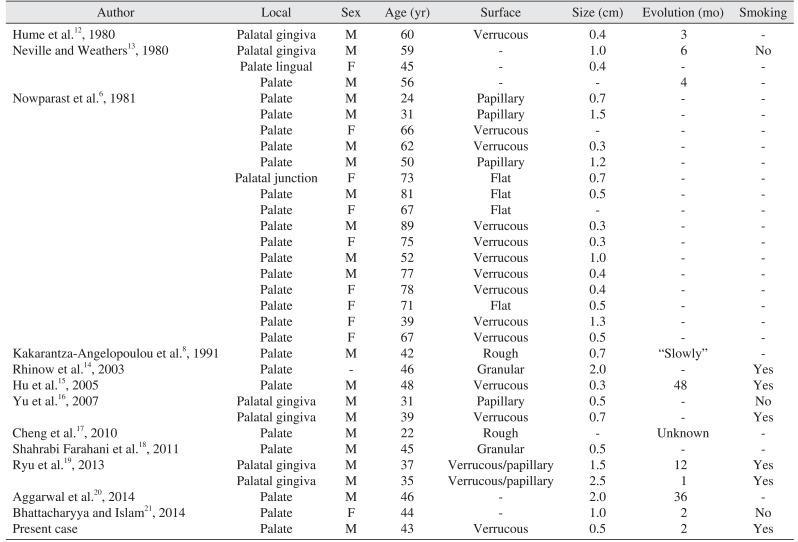
We found 12 studies in the literature reporting verruciform xanthoma in the hard palate
6812131415161718192021. Articles without clinical information, immunohistochemical studies, multiple lesions, or involving the soft palate were excluded. The results are shown in
Table 1.
Table 1
Clinical features of verruciform xanthoma in the hard palate
|
Author |
Local |
Sex |
Age (yr) |
Surface |
Size (cm) |
Evolution (mo) |
Smoking |
|
Hume et al.12, 1980 |
Palatal gingiva |
M |
60 |
Verrucous |
0.4 |
3 |
- |
|
Neville and Weathers13, 1980 |
Palatal gingiva |
M |
59 |
- |
1.0 |
6 |
No |
|
Palate lingual |
F |
45 |
- |
0.4 |
- |
- |
|
Palate |
M |
56 |
- |
- |
4 |
- |
|
Nowparast et al.6, 1981 |
Palate |
M |
24 |
Papillary |
0.7 |
- |
- |
|
Palate |
M |
31 |
Papillary |
1.5 |
- |
- |
|
Palate |
F |
66 |
Verrucous |
- |
- |
- |
|
Palate |
M |
62 |
Verrucous |
0.3 |
- |
- |
|
Palate |
M |
50 |
Papillary |
1.2 |
- |
- |
|
Palatal junction |
F |
73 |
Flat |
0.7 |
- |
- |
|
Palate |
M |
81 |
Flat |
0.5 |
- |
- |
|
Palate |
F |
67 |
Flat |
- |
- |
- |
|
Palate |
M |
89 |
Verrucous |
0.3 |
- |
- |
|
Palate |
F |
75 |
Verrucous |
0.3 |
- |
- |
|
Palate |
M |
52 |
Verrucous |
1.0 |
- |
- |
|
Palate |
M |
77 |
Verrucous |
0.4 |
- |
- |
|
Palate |
F |
78 |
Verrucous |
0.4 |
- |
- |
|
Palate |
F |
71 |
Flat |
0.5 |
- |
- |
|
Palate |
F |
39 |
Verrucous |
1.3 |
- |
- |
|
Palate |
F |
67 |
Verrucous |
0.5 |
- |
- |
|
Kakarantza-Angelopoulou et al.8, 1991 |
Palate |
M |
42 |
Rough |
0.7 |
“Slowly” |
- |
|
Rhinow et al.14, 2003 |
Palate |
- |
46 |
Granular |
2.0 |
- |
Yes |
|
Hu et al.15, 2005 |
Palate |
M |
48 |
Verrucous |
0.3 |
48 |
Yes |
|
Yu et al.16, 2007 |
Palatal gingiva |
M |
31 |
Papillary |
0.5 |
- |
No |
|
Palatal gingiva |
M |
39 |
Verrucous |
0.7 |
- |
Yes |
|
Cheng et al.17, 2010 |
Palate |
M |
22 |
Rough |
- |
Unknown |
- |
|
Shahrabi Farahani et al.18, 2011 |
Palate |
M |
45 |
Granular |
0.5 |
- |
- |
|
Ryu et al.19, 2013 |
Palatal gingiva |
M |
37 |
Verrucous/papillary |
1.5 |
12 |
Yes |
|
Palatal gingiva |
M |
35 |
Verrucous/papillary |
2.5 |
1 |
Yes |
|
Aggarwal et al.20, 2014 |
Palate |
M |
46 |
- |
2.0 |
36 |
- |
|
Bhattacharyya and Islam21, 2014 |
Palate |
F |
44 |
- |
1.0 |
2 |
No |
|
Present case |
Palate |
M |
43 |
Verrucous |
0.5 |
2 |
Yes |

Men were twice as likely to be affected than women (2:1). Lesions were normally small (average size of lesions 0.85 cm); however, three OVX lesions were 2 cm or more. The lesion surface was mostly verrucous. Older patients in the 40 to 90 year age range (mean age, 54 years) were significantly more frequently affected than younger patients. Almost 63% of the patients were smokers (5 of the 8 cases).
This case reinforces that OVX in the hard palate presents as small, painless lesions and affects men below 50 years. The influence of smoking habits is discussed below.
The diagnostic hallmark of OVX is the presence of lipidladen macrophages in the connective tissue between the epithelial ridges
2416. An extensive immunohistochemical study showed that foam cells are of monocyte-macrophage lineage based on intense cytoplasmic positivity for anti-CD68 and cathepsin B
9. Rawal et al.
10 demonstrated that macrophages present in OVX are primarily reparative and resident, supporting the chronic reactive nature of OVX. In this case, a large number of xanthoma cells with clear cytoplasm and pyknotic nucleus were found. These cells were positive for CD6, confirming them as macrophages. The anti-KI-67 antibody was restricted to the basal layer of the epithelium, showing that the lesion was not malignant.
The pathogenesis of OVX remains unknown. Most patients with OVX do not have hyperlipidemia
22. The mechanism of the accumulation of lipid-containing foam cells in the submucosa or dermis is also not clear
2.
An ultrastructural and immunohistochemical study showed that, after the development of OVX, oxidized low-density lipoprotein (ox-LDL) induces foam cell necrosis, and resident macrophages clean the debris and cyclically perpetuate VX
23. Ultrastructural and in situ hybridization have not shown an association between HPV and OVX, excluding a viral etiology for this lesion
24.
Metabolic abnormalities of lipids and lymphedema are associated with nonmucosal cutaneous verruciform xanthoma
325. The obstruction of lymphatic vessels results in accumulation of lipids in the connective tissue, and these lipids are scavenged by macrophages, producing xanthomas in patients with lymphedema
25.
Patients with CHILD syndrome show inactivity of an enzyme related with cholesterol synthesis and interestingly have been reported to have concomitant verruciform xanthoma
26.
The association of systemic diseases with cutaneous verruciform xanthoma but not OVX indicates that local trauma and inflammation are the most likely causes of OVX, since the epithelial cell degeneration caused by this condition releases lipid material that is scavenged by local macrophages. Studies have shown that the macrophages present in OVX are resident, mature, chronic inflammatory macrophages, confirming that is a chronic, slow-growing inflammatory lesion
10.
These findings are consistent with the influence of smoking habits in the pathogenesis of OVX. Local trauma caused by cigarettes, especially in the hard palate, may be associated with OVX development. However, further studies need to be performed to confirm this association.
We did not find specific OVX surveys by location in the literature. The hard palate is the second most affected area, and local trauma caused by smoking can be a cause of this lesion. Knowledge of uncommon lesions in the mouth is extremely important for a correct diagnosis and treatment.
Go to :








 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download