This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
To evaluate the results of elective neck dissection versus those of observation in the treatment of early stage oral squamous cell carcinoma and to identify factors related to recurrence and survival.
Materials and Methods
This was a retrospective study of 52 patients who underwent elective neck dissection and 27 who did not receive neck dissection.
Results
In survival analyses, elective neck dissection showed a benefit in overall recurrence (P=0.027), especially in stage I patients (P=0.024). With regard to survival, the benefit was statistically insignificant (P=0.990). In multivariable analysis, overall recurrence was independently related to poor histologic grade (odds ratio [OR]=9.65, P=0.006), and cancer-specific death was independently related to advanced age (OR=6.3, P=0.022), higher clinical T stage (OR=15.2, P=0.01), and poorly differentiated histologic grade (OR=6.6, P=0.025).
Conclusion
Though there was lower recurrence in the elective neck dissection group, there were no statistically significant results on survival. The characteristics of the tumor itself, such as clinical T stage and poor histologic grade, may be more important in cancer-specific survival.
Keywords: Oral cancer, Squamous cell carcinoma, Neck dissection, Survival analysis, Survival rate
I. Introduction
The initial management of the neck in early oral squamous cell carcinoma (OSCC) with clinically negative neck nodes (clinical T stage [cT] 1 or 2 and clinical N stage [cN] 0) remains a controversy. Before the 1990s, the traditional policy for management of the clinically negative neck in early OSCC was generally a ‘wait-and-see’ or observation (OBS) policy, unless the neck was being opened for other reasons such as better access to the primary tumor, reconstruction requirements, or delaying neck dissection until cervical metastases was clinically evident.
The incidence of occult regional lymph node metastasis of OSCC varies from 6% to 46%, according to previous reports
1. Once regional metastases have occurred, the 5-year survival rate for patients with oral cancer decreases by one-half relative to that of patients with early-stage disease
23. In view of the high incidence of nodal recurrence of the observed neck, prophylactic neck dissection has been advocated as a routine management protocol of N0 neck
4.
The results of the few prospective randomized studies and a retrospective study on the benefit of prophylactic neck treatment have been inconclusive. The studies failed to find statistically significant differences in prognoses between the groups of patients under OBS without initial neck dissection and those groups initially managed with neck dissection
567.
The aim of this study was to retrospectively review and compare the outcomes between patients under OBS and patients who underwent elective neck dissection (END) at initial surgery and to identify factors that affect locoregional control and survival.
II. Materials and Methods
This retrospective study included 215 OSCC patients who underwent surgical treatment at Department of Oral and Maxillofacial Surgery, Yonsei University Dental Hospital (Seoul, Korea) from 1990 to 2012, based on a screening of medical records. This study was approved by the regional Ethical Review Board of Yonsei University Dental Hospital Institutional Review Board (IRB No. 2-2014-0032). Patients with recurred tumor, second primary tumor, metastatic tumor, and patients who underwent salvage surgery were excluded. Patients who had clinically T3 or T4 tumor were also excluded. Patients with positive neck nodes on physical exam or imaging studies, including computed tomography (CT), magnetic resonance imaging, positron emission tomography (PET), PET-CT, and ultrasonography, were excluded. After the selection process, 79 patients were suitable for analysis. Among them, 52 patients underwent END and 27 patients did not receive neck dissection and underwent OBS only.
Following the policy of most institutions, OBS was applied when there was no evidence of neck node metastasis, and END was applied to patients with suspicion of neck node metastasis despite negative results.
In the OBS group, occult cervical metastasis was defined as a neck recurrence during follow-up, without failure at the primary site
89. Cervical metastases in patients with recurrent primary tumor were not considered occult metastases because the nodal spread may have occurred after the initial treatment
89. In the END group, occult disease was defined as the presence of microscopic disease on the histopathologic examination of neck dissection specimens
89.
Survival curves were plotted using the Kaplan-Meier method and compared using the log-rank test. Chi-squared test or Fisher exact test was used for categorical data analysis. Univariable analyses were performed to find factors affecting recurrence. For multivariable analyses to find independently related factors of recurrence and survival, the Cox proportional hazard model was used. Differences were considered significant for P-value <0.05. All statistical analyses were performed with PASW Statistics software version 18.0 (IBM Co., Armonk, NY, USA).
III. Results
1. Characteristics of the study population
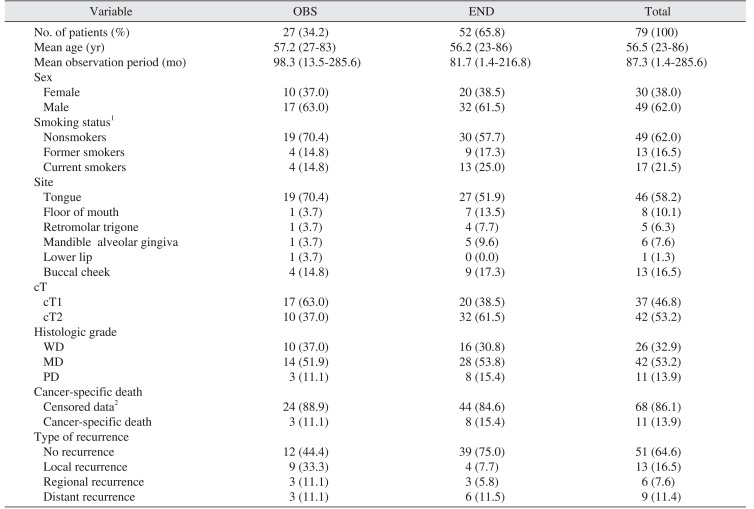
The patients were divided into two groups: OBS (n=27, 34.2%) and END (n=52, 65.8%). The median age of the entire group was 59 years. The OBS period varied from 1.4 months to 285.6 months with a mean of 87.3 months. Regarding the site of primary lesion, 46 (58.2%) were from tongue, 13 (16.5%) from buccal cheek, 8 (10.1%) from floor of mouth, 6 (7.6%) from mandibular alveolar gingiva, 5 (6.3%) from retromolar trigone, and 1 (1.3%) was from maxillary alveolar gingiva. The stages were cT1 and cT2 in 17 patients (63.0%) and 10 patients (37.0%) in the OBS group, respectively, and 20 patients (38.5%) and 32 patients (61.5%) in the END group. In total, there were 37 patients (46.8%) with cT1 stage and 42 patients (53.2%) with cT2 stage. The characteristics of the study population are shown in
Table 1.
2. Characteristics of nodal metastasis
Characteristics of nodal metastasis were investigated in the END group. Forty-two cases (42/52, 80.8%) were pathologically confirmed as negative for neck node metastasis (pN0). Among the remaining 10 cases (10/52, 19.2%), 8 (15.4%) were pN1, and 2 (3.8%) were pN2. Forty-one patients (78.8%) underwent ipsilateral selective neck dissection (SND). Six patients (11.5%) underwent bilateral SND, and 5 patients (9.6%) underwent modified radical neck dissection.
3. Survival analyses
The END group showed a statistically significant benefit in disease-free survival (
P=0.027;
Fig. 1), especially in stage I patients (
P=0.024).(
Fig. 2) However, there was no statistically significant difference in regional recurrence-free survival or cancer-specific survival between OBS and END groups. (
Fig. 3,
4)
4. Occult metastasis
Occult cervical nodal metastases were found in 13 cases (13/79, 16.5%), including neck recurrence in 3 cases of the OBS group (3/27, 11.1%) and pathologically positive metastases in 10 cases of the END group (10/52, 19.2%). In univariable analysis, occult metastasis was related to higher odds of cancer-specific death (odds ratio [OR]=6.25, P<0.01).
5. Factors of overall recurrence
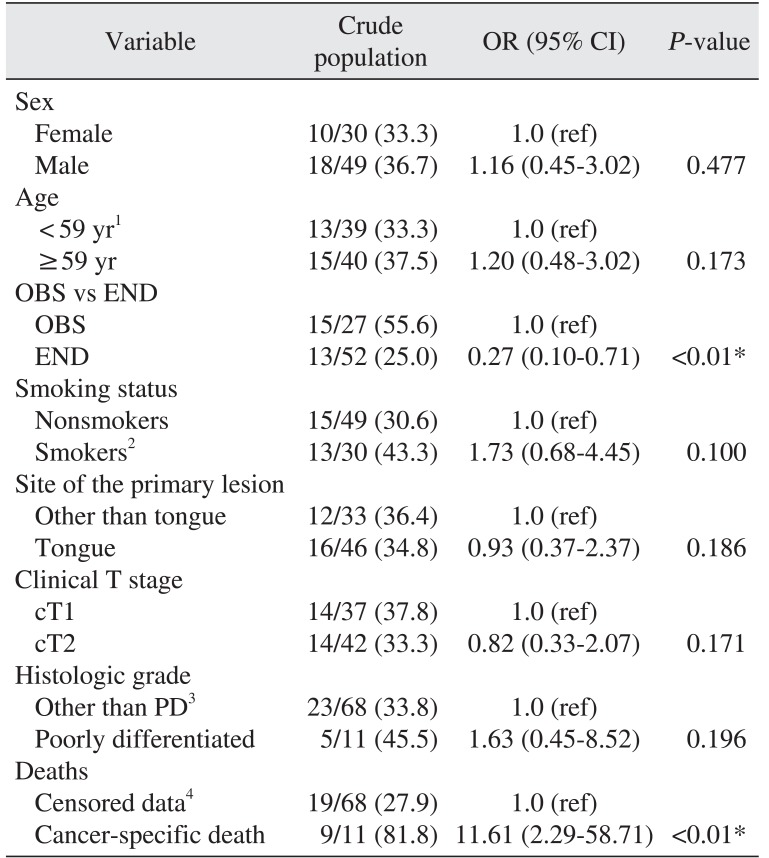
To determine the factors related to overall recurrence, we performed univariable and multivariable analyses. In univariable analyses, the END group was correlated with lower overall recurrence (OR=0.27,
P=0.006).(
Table 2) Cancer-specific death was related to higher odds of overall recurrence (OR=11.61,
P<0.01).(
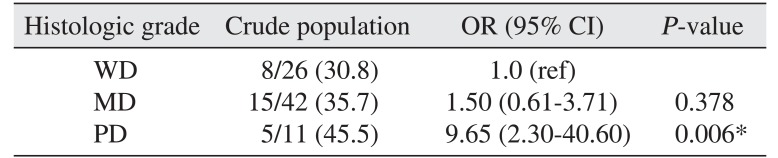
Table 2) In multivariable analysis, poor histologic grade was independently related to overall recurrence (OR=9.65,
P<0.01).(
Table 3)
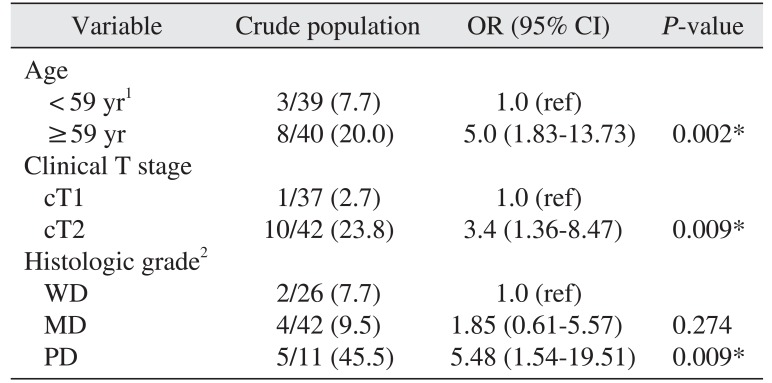
6. Factors of cancer-specific death
In a multivariable analysis, advanced age (OR=6.3,
P=0.022), higher clinical T stage (OR=15.2,
P=0.01), and poorly differentiated histologic grade (OR=6.6,
P=0.025) were independently related to cancer-specific death.(
Table 4)
IV. Discussion
A therapeutic neck dissection is obviously necessary when clinically invaded nodes are present
10. The presence of regional neck metastases is widely accepted as a major determinant of prognosis in patients with OSCC
1112. If the probability of neck metastases is high, a neck dissection will decrease the risk of regional recurrence. However, if the probability of neck metastases is low, neck dissection constitutes overtreatment, where the morbidity of the neck procedure only decreases quality of life while increasing functional deficits. While the problem could be solved if it were possible to predict the risk of neck metastases, such prediction has been difficult to introduce and apply in clinical practice. Finally, there is little published guidance about management of the N0 neck in OSCC
13.
Patients with cT1N0 and cT2N0 OSCC have been reported to have occult metastases in 13% to 33% and 37% to 53% of cases, respectively, at the time of diagnosis
14. In this study, the overall occult cervical nodal metastatic rate was 16.5% (13/79). The rates for cT1N0 and cT2N0 in this study were 8.1% (3/37) and 23.8% (10/42), respectively. Though the occult metastasis rate is relatively low in our study, the tendency toward comparably higher incidence of occult metastasis in T2 compared to T1, as well as ipsilateral neck being the common site of recurrence, seem congruent with results from previous studies
9.
An interesting result of our study is that the END group was related to lower recurrence, but did not seem related to better survival. Of course, we should carefully interpret these results since this study was a retrospective analysis and not a double-blind prospective randomized control trial, so a selection bias (i.e., predilection of END for advanced T stages) may have influenced the results. Most studies have focused on the rate of occult metastasis and related factors to demonstrate the necessity of END. Our results tentatively suggest that END may lower the rate of recurrence, but not necessarily improve survival, and that the characteristics of the tumor itself, such as clinical T stage and poor histologic grade, may be important for survival. These results may be helpful when determining the necessity of END for early stage OSCC.
Previous studies have suggested other factors that influence final outcome and survival of cancer treatment. Eicher et al.
15 recommended END for patients with moderately or poorly differentiated SCC, radiological or histological signs of bony invasion, and tumors in the mandibular symphyseal region
16. Ogura et al.
17 examined mandibular bony invasion using dental CT and found it unfavorable as a prognostic indicator of 5-year survival.
Feng et al.
11 reported that the follow-up compliance of patient populations was the vital factor in adopting the OBS strategy for the cN0 neck. They stated that early detection of regional recurrence led to a 100% cervical salvage rate irrespective of T stage, the salvage rate otherwise dropping as remarkably low as <30.0%
11. They concluded that END should be recommended as first-line management for all intermediate and advanced stage patients, with the exception of patients with stage T1 tumors, who have a low risk of nodal metastasis and for whom OBS may be an acceptable alternative to END if the patients strictly comply with a cancer surveillance protocol
11.
V. Conclusion
For better survival of early OSCC patients without clinically evident neck node metastasis, characteristics of the tumor itself, such as advanced T stage and poor histologic grade, may be equally or more important than the treatment modality of the neck. The follow-up compliance of the patients must be guaranteed to adopt the OBS strategy for the cN0 neck.
Acknowledgements
Authorship and Contributions: Study concepts and design (Ba-Da Lee, In-Ho Cha); data acquisition (Woong Nam, Hyung Jun Kim, In-Ho Cha); data analysis and interpretation (Jung Hwan Lim, Dong Wook Kim); statistical analysis (Ba-Da Lee, Dong Wook Kim); manuscript preparation and editing (Jung-Hyun Park, Dong Wook Kim); manuscript review (Woong Nam, Hyung Jun Kim, In-Ho Cha).
References
1. Byers RM, El-Naggar AK, Lee YY, Rao B, Fornage B, Terry NH, et al. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck. 1998; 20:138–144. PMID:
9484945.

2. Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002; 24:731–736. PMID:
12203797.

3. Pimenta Amaral TM, Da Silva Freire AR, Carvalho AL, Pinto CA, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol. 2004; 40:780–786. PMID:
15288831.

4. Werning JW, Heard D, Pagano C, Khuder S. Elective management of the clinically negative neck by otolaryngologists in patients with oral tongue cancer. Arch Otolaryngol Head Neck Surg. 2003; 129:83–88. PMID:
12525200.

5. Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, et al. Prospective randomized study of selective neck dissection versus observation for N0 neck of early tongue carcinoma. Head Neck. 2009; 31:765–772. PMID:
19408291.

6. Fakih AR, Rao RS, Borges AM, Patel AR. Elective versus therapeutic neck dissection in early carcinoma of the oral tongue. Am J Surg. 1989; 158:309–313. PMID:
2802032.

7. Vandenbrouck C, Sancho-Garnier H, Chassagne D, Saravane D, Cachin Y, Micheau C. Elective versus therapeutic radical neck dissection in epidermoid carcinoma of the oral cavity: results of a randomized clinical trial. Cancer. 1980; 46:386–390. PMID:
6992980.

8. Keski-Säntti H, Atula T, Törnwall J, Koivunen P, Mäkitie A. Elective neck treatment versus observation in patients with T1/T2 N0 squamous cell carcinoma of oral tongue. Oral Oncol. 2006; 42:96–101. PMID:
16256414.

9. Liu TR, Chen FJ, Yang AK, Zhang GP, Song M, Liu WW, et al. Elective neck dissection in clinical stage I squamous cell carcinoma of the tongue: does it improve regional control or survival time? Oral Oncol. 2011; 47:136–141. PMID:
21216182.

10. Lubek J, El-Hakim M, Salama AR, Liu X, Ord RA. Gingival carcinoma: retrospective analysis of 72 patients and indications for elective neck dissection. Br J Oral Maxillofac Surg. 2011; 49:182–185. PMID:
20462676.

11. Feng Z, Li JN, Li CZ, Guo CB. Elective neck dissection versus observation for cN0 neck of squamous cell carcinoma primarily located in the maxillary gingiva and alveolar ridge: a retrospective study of 129 cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013; 116:556–561. PMID:
24119520.

12. Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008; 371:1695–1709. PMID:
18486742.

13. Kowalski LP, Sanabria A. Elective neck dissection in oral carcinoma: a critical review of the evidence. Acta Otorhinolaryngol Ital. 2007; 27:113–117. PMID:
17883186.
14. Fasunla AJ, Greene BH, Timmesfeld N, Wiegand S, Werner JA, Sesterhenn AM. A meta-analysis of the randomized controlled trials on elective neck dissection versus therapeutic neck dissection in oral cavity cancers with clinically node-negative neck. Oral Oncol. 2011; 47:320–324. PMID:
21459661.

15. Eicher SA, Overholt SM, el-Naggar AK, Byers RM, Weber RS. Lower gingival carcinoma. Clinical and pathologic determinants of regional metastases. Arch Otolaryngol Head Neck Surg. 1996; 122:634–638. PMID:
8639295.

16. Overholt SM, Eicher SA, Wolf P, Weber RS. Prognostic factors affecting outcome in lower gingival carcinoma. Laryngoscope. 1996; 106:1335–1339. PMID:
8914897.

17. Ogura I, Kurabayashi T, Amagasa T, Okada N, Sasaki T. Mandibular bone invasion by gingival carcinoma on dental CT images as an indicator of cervical lymph node metastasis. Dentomaxillofac Radiol. 2002; 31:339–343. PMID:
12424630.

Fig. 1
Disease-free survival. There was a statistically significant difference between observation (OBS) and elective neck dissection (END) groups (P=0.027).

Fig. 2
Disease-free survival in stage I and II patients. In stage I patients, there was a significant difference between observation (OBS) and elective neck dissection (END) groups (stage I, P=0.024; stage II, P=0.373). Statistical analysis by Kaplan-Meier survival estimates and log-rank test.
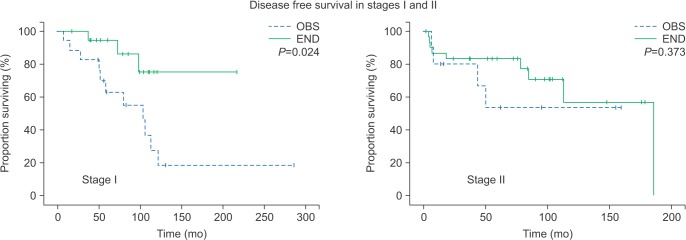
Fig. 3
Regional recurrence-free survival. No significant difference between observation (OBS) and elective neck dissection (END) groups (P=0.990). Statistical analysis by Kaplan-Meier survival estimates and log-rank test.

Fig. 4
Cancer-specific survival. No significant difference between observation (OBS) and elective neck dissection (END) groups (P=0.990). Statistical analysis by Kaplan-Meier survival estimates and log-rank test.

Table 1
Characteristics of the study population
|
Variable |
OBS |
END |
Total |
|
No. of patients (%) |
27 (34.2) |
52 (65.8) |
79 (100) |
|
Mean age (yr) |
57.2 (27-83) |
56.2 (23-86) |
56.5 (23-86) |
|
Mean observation period (mo) |
98.3 (13.5-285.6) |
81.7 (1.4-216.8) |
87.3 (1.4-285.6) |
|
Sex |
|
|
|
|
Female |
10 (37.0) |
20 (38.5) |
30 (38.0) |
|
Male |
17 (63.0) |
32 (61.5) |
49 (62.0) |
|
Smoking status1
|
|
|
|
|
Nonsmokers |
19 (70.4) |
30 (57.7) |
49 (62.0) |
|
Former smokers |
4 (14.8) |
9 (17.3) |
13 (16.5) |
|
Current smokers |
4 (14.8) |
13 (25.0) |
17 (21.5) |
|
Site |
|
|
|
|
Tongue |
19 (70.4) |
27 (51.9) |
46 (58.2) |
|
Floor of mouth |
1 (3.7) |
7 (13.5) |
8 (10.1) |
|
Retromolar trigone |
1 (3.7) |
4 (7.7) |
5 (6.3) |
|
Mandible alveolar gingiva |
1 (3.7) |
5 (9.6) |
6 (7.6) |
|
Lower lip |
1 (3.7) |
0 (0.0) |
1 (1.3) |
|
Buccal cheek |
4 (14.8) |
9 (17.3) |
13 (16.5) |
|
cT |
|
|
|
|
cT1 |
17 (63.0) |
20 (38.5) |
37 (46.8) |
|
cT2 |
10 (37.0) |
32 (61.5) |
42 (53.2) |
|
Histologic grade |
|
|
|
|
WD |
10 (37.0) |
16 (30.8) |
26 (32.9) |
|
MD |
14 (51.9) |
28 (53.8) |
42 (53.2) |
|
PD |
3 (11.1) |
8 (15.4) |
11 (13.9) |
|
Cancer-specific death |
|
|
|
|
Censored data2
|
24 (88.9) |
44 (84.6) |
68 (86.1) |
|
Cancer-specific death |
3 (11.1) |
8 (15.4) |
11 (13.9) |
|
Type of recurrence |
|
|
|
|
No recurrence |
12 (44.4) |
39 (75.0) |
51 (64.6) |
|
Local recurrence |
9 (33.3) |
4 (7.7) |
13 (16.5) |
|
Regional recurrence |
3 (11.1) |
3 (5.8) |
6 (7.6) |
|
Distant recurrence |
3 (11.1) |
6 (11.5) |
9 (11.4) |
Table 2
Univariable analysis of overall recurrence
|
Variable |
Crude population |
OR (95% CI) |
P-value |
|
Sex |
|
|
|
|
Female |
10/30 (33.3) |
1.0 (ref) |
|
|
Male |
18/49 (36.7) |
1.16 (0.45-3.02) |
0.477 |
|
Age |
|
|
|
|
< 59 yr1
|
13/39 (33.3) |
1.0 (ref) |
|
|
≥59 yr |
15/40 (37.5) |
1.20 (0.48-3.02) |
0.173 |
|
OBS vs END |
|
|
|
|
OBS |
15/27 (55.6) |
1.0 (ref) |
|
|
END |
13/52 (25.0) |
0.27 (0.10-0.71) |
<0.01*
|
|
Smoking status |
|
|
|
|
Nonsmokers |
15/49 (30.6) |
1.0 (ref) |
|
|
Smokers2
|
13/30 (43.3) |
1.73 (0.68-4.45) |
0.100 |
|
Site of the primary lesion |
|
|
|
|
Other than tongue |
12/33 (36.4) |
1.0 (ref) |
|
|
Tongue |
16/46 (34.8) |
0.93 (0.37-2.37) |
0.186 |
|
Clinical T stage |
|
|
|
|
cT1 |
14/37 (37.8) |
1.0 (ref) |
|
|
cT2 |
14/42 (33.3) |
0.82 (0.33-2.07) |
0.171 |
|
Histologic grade |
|
|
|
|
Other than PD3
|
23/68 (33.8) |
1.0 (ref) |
|
|
Poorly differentiated |
5/11 (45.5) |
1.63 (0.45-8.52) |
0.196 |
|
Deaths |
|
|
|
|
Censored data4
|
19/68 (27.9) |
1.0 (ref) |
|
|
Cancer-specific death |
9/11 (81.8) |
11.61 (2.29-58.71) |
<0.01* |
Table 3
Multivariable analysis of overall recurrence
|
Histologic grade |
Crude population |
OR (95% CI) |
P-value |
|
WD |
8/26 (30.8) |
1.0 (ref) |
|
|
MD |
15/42 (35.7) |
1.50 (0.61-3.71) |
0.378 |
|
PD |
5/11 (45.5) |
9.65 (2.30-40.60) |
0.006*
|
Table 4
Multivariable analysis of cancer-specific death
|
Variable |
Crude population |
OR (95% CI) |
P-value |
|
Age |
|
|
|
|
< 59 yr1
|
3/39 (7.7) |
1.0 (ref) |
|
|
≥59 yr |
8/40 (20.0) |
5.0 (1.83-13.73) |
0.002*
|
|
Clinical T stage |
|
|
|
|
cT1 |
1/37 (2.7) |
1.0 (ref) |
|
|
cT2 |
10/42 (23.8) |
3.4 (1.36-8.47) |
0.009*
|
|
Histologic grade2
|
|
|
|
|
WD |
2/26 (7.7) |
1.0 (ref) |
|
|
MD |
4/42 (9.5) |
1.85 (0.61-5.57) |
0.274 |
|
PD |
5/11 (45.5) |
5.48 (1.54-19.51) |
0.009*
|












 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download