Abstract
Objectives
The aim of the present study was to evaluate changes in the management and 5-year survival rates of patients with oral cancer in our department over a 30-year period.
Materials and Methods
We investigated the patient distributions, treatment methods, method of neck dissection according to cancer stage, and 5-year survival rates for 700 oral cancer patients over the periods of 1982–1996 (256 patients), 1999–2006 (248 patients), and 2007–2011 (196 patients).
Results
Stage IV patients were the largest group in all of the time periods evaluated. Although surgery and radiotherapy were the most common methods in all periods (over 50%), the prevalence of patients who underwent concomitant chemoradiotherapy increased from 7.0% to 16.2%. The use of radical neck dissection decreased from 43.0% to 5.3%, while conservative surgical methods increased from 24.1% to 76.3%. Lastly, the overall 5-year survival rate increased from 31.6% to 63.5% during the study period.
Conclusion
Although the 5-year survival rate reached the same level as that of other developed countries during the course of our study, most patients continue to come to the hospital with stage IV disease. In order to increase the 5-year survival rate of oral carcinoma, it may be necessary to improve public education and social efforts relevant to early diagnosis.
The oldest medical record of carcinoma is an ancient Egyptian case of breast cancer1, while the oldest medical record of oral carcinoma concerns an ulcerative lesion at the gums and the tongue described in 1500 A.D. Throughout history clinicians and researchers have developed new drugs, conceived new tools, and designed new surgical methods to treat cancer patients and improve 5-year survival rates. The first partial glossectomy and removal of the submandibular gland under general anesthesia was performed by Dr. John Collins Warren in 18462. Radiotherapy was developed after successful pain relief in pharyngeal cancer by Dr. Voigt in 1896345. Chemotherapy and immunotherapy were developed after the Second World War owing to the development of poison gas6. Regarding surgical strategies for cervical lymph nodes, there has been a transition from early radical neck dissection (RND) to selective neck dissection. However, controversy continues to surround N0 oral carcinomas78.
Currently, there is no difference between the outcomes of surgery and radiotherapy alone in early-stage oral carcinomas. However, surgical treatment is generally recommended for early and small-sized tumors because treatment of these early-stage lesions remain results in a fewer esthetic problems and is associated with fewer functional complications as compared to radiotherapy. On the other hand, with respect to advanced-stage oral carcinomas, the combination of surgery, radiotherapy, and/or chemotherapy is more effective than single-modality therapy9.
Although various and new treatment modalities are being applied to oral carcinomas aimed at life extension, there have been no remarkable increases in 5-year survival rates over the past 30 years10. According to data from the National Cancer Institute, the 5-year survival rates of oral and pharyngeal cancer were 52.6%, 54.4%, 56.5%, and 62.2% between 1970–1975, 1980–1985, 1993–1997, and 2003–2009, repectively11. Similarly, data from Cancer Research UK reported that the 5-year survival rates of oral cancer were 48.1% and 50% between 1986–1990 and 1996–1999, respectively12.
The aims of the present study were to evaluate changes in the management and 5-year survival rates of patients with oral cancer at our department over 30 years. In addition, we sought to identify new means of improving the 5-year survival rates and quality of life of patients with oral cancer.
We investigated a total of 700 patients with oral cancer. The study population consisted of 196 newly investigated patients who were treated from January 2007 to December 2011, as well as 504 previously investigated patients who had been diagnosed or treated between 1982–1996 (256 patients)13 or 1999–2006 (248 patients)14. All patients were diagnosed and/or treated at the Department of Oral and Maxillofacial Surgery, Kyungpook National University School of Dentistry (Daegu, Korea).
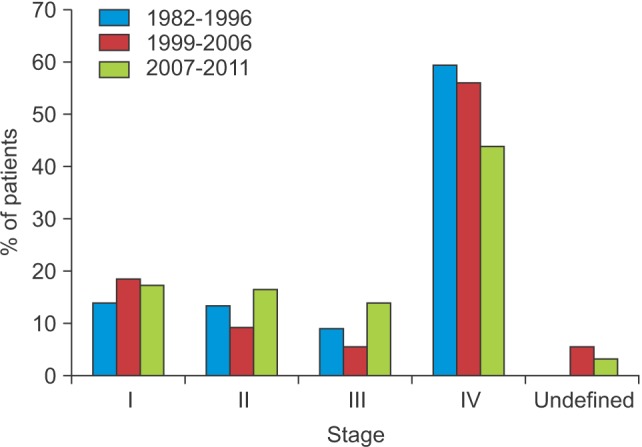
The numbers of patients with tumor stage I, II, III, and IV disease were 18 (15.1%), 17 (14.3%), 12 (10.1%), and 72 (60.5%), respectively.(Fig. 1)
There were 48 (19.4%), 26 (10.5%), 16 (6.5%), and 142 (57.3%) patients with stage I, II, III, and IV disease, respectively.(Fig. 1)
There were 37 (18.9%), 33 (16.8%), 26 (13.3%), and 80 (40.8%) patients with stage I, II, III, and IV disease, respectively.(Fig. 1)
Disease stage could not be determined for 20 patients (10.2%), due to inadequate medical records. Patients with stage IV disease comprised the largest proportion of the study population, while patients with stage III disease comprised the smallest. These results were consistent with previous investigations 1314.
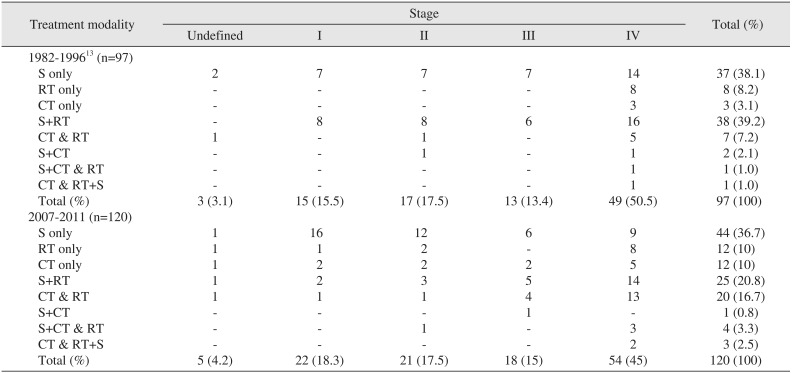
Among the 15 patients with stage I disease, seven (46.7%) received surgical treatment alone and eight (53.3%) received additional postoperative radiotherapy (PORT). Out of the 17 patients with stage II disease, seven (41.2%) received surgery alone, eight (47.1%) received PORT, one (5.9%) received postoperative chemotherapy (POCT), and one (5.9%) received postoperative concomitant chemoradiotherapy (CCRT). Of the 13 patients with stage III disease, seven (53.8%) underwent surgery and six (46.2%) received PORT. Among 49 patients with stage IV disease, 14 (28.6%) underwent surgery alone, 16 (32.7%) received additional PORT, one (2.0%) received POCT, and one received CCRT. Radiotherapy alone was performed for eight patients (16.3%), while three patients (6.1%) received chemotherapy alone and one (2.0%) received CCRT. Of the three patients with an unclassified disease stage, two received surgery alone and the remaining patient received CCRT. Among all patients, 77.3% underwent surgery or PORT, which were the most commonly chosen treatment methods for oral cancer in this period.(Table 1; 1982–1996)
Among stage I cases, 16 patients (72.8%) received surgery alone, one patient (4.5%) received radiotherapy, two patients (9.1%) received chemotherapy, two received PORT, and one received postoperative CCRT. Among stage II cases (n=21), 12 patients (57.1%) underwent surgery alone, two patients (9.5%) received radiotherapy alone, and two patients received chemotherapy. Three patients (14.3%) received surgery and PORT, one patient (4.8%) received CCRT, and one patient received surgery and postoperative CCRT. Among 18 patients with stage III, six (33.3%) underwent surgery alone, two (9.5%) received surgery and PORT, and one (5.6%) received surgery and POCT. Four patients (22.2%) received CCRT only. Among stage IV cases (n=54), nine patients (16.7%) underwent surgery alone, eight patients (14.8%) received radiotherapy alone, and five patients (9.3%) received chemotherapy alone. A total of 14 patients (25.8%) underwent surgery and PORT while three patients (24.1%) underwent surgery followed by postoperative CCRT. Thirteen patients (24.1%) received only CCRT. Two patients (3.7%) received preoperative CCRT and surgery. Among patients with an undetermined disease stage, a single-modality treatment of surgery, radiotherapy, or chemotherapy was provided to each of three patients. Lastly, PORT following surgery was provided to one patient and CCRT alone was provided to one patient.(Table 1; 2007–2011)
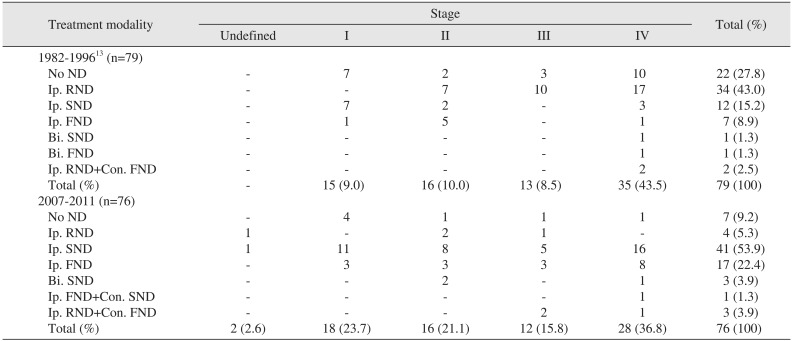
There were 15 patients with stage I disease, of which seven (46.7%) did not undergo neck surgery, seven underwent ipsilateral supraomohyoid neck dissection (Ip. SND), and one (6.7%) underwent ipsilateral functional neck dissection (Ip. FND). Of 16 patients with stage II disease, two (12.5%) did not undergo neck surgery, seven (43.8%) underwent Ip. RND, two (12.5%) underwent Ip. SND, and five (31.3%) underwent Ip. FND. Among 13 patients with stage III disease, three (23.1%) did not undergo neck dissection and ten (76.9%) received Ip. RND. Ten patients (28.6%) did not undergo neck dissection, 17 patients (48.6%) underwent Ip. RND, three patients (8.6%) underwent Ip. SND, one patient (2.9%) underwent Ip. FND, one patient underwent bilateral SND (Bi. SND), and two patients (5.7%) underwent both Ip. RND and contralateral FND (Con. FND).(Table 2; 1982–1996)
Out of 18 patients with stage I disease, four (22.2%) did not undergo neck dissection, 11 (61.1%) underwent Ip. SND, and three (16.7%) underwent Ip. FND. Among stage II cases, one patient (6.3%) did not undergo neck dissection, two patients (12.4%) underwent Ip. RND, eight patients (50.0%) underwent Ip. SND, three patients (18.8%) underwent Ip. FND, and two patients underwent Bi. SND. Among 12 patients with stage III disease, there was one patient (8.3%) in which neck dissection was not performed. Further, Ip. RND was performed in one patient, Ip. SND was performed in five patients (41.7%), Ip. FND was performed in three patients (24.0%), and Ip. RND+Con. FND was performed in two patients (16.7%). Of 28 patients with stage IV, one (3.6%) did not undergo neck dissection, 16 (57.1%) received Ip. SND, and eight (28.5%) received Ip. FND, while Bi. SND, Ip. FND+Con. SND, and Ip. RND+Con. FND were used to treat one patient each. Finally, there were five patients who were not classified.
Twelve of the 38 patients who were treated between 1982–1990 survived (31.6%). On the other hand, the 5-year survival rate between 1991–1996 was 54.0% as estimated using the life table method. Finally, the Kaplan-Meier 5-year survival rates between 1999–2006 and 2007–2011 were 57.7% and 63.5%, respectively.
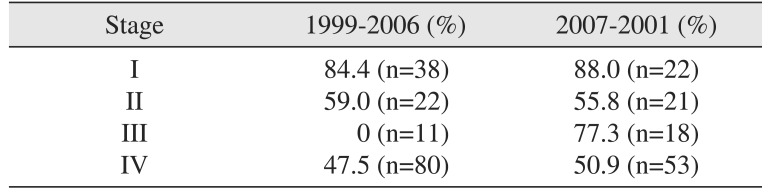
Between 1999–2006, the 5-year survival rates for stage I, I, II, and IV disease were 84.4%, 59.0%, 0%, and 47.5%, respectively. On the other hand, between 2007–2011, the 5-year survival rates for patients with stage I, I, II, and IV disease were 88.0%, 55.8%, 77.3%, and 50.9%, respectively.(Fig. 2, Table 3)
It is well known that the time between tumor development and diagnosis is one of the most important factors that influence the prognosis of malignant tumors15. Consequently, early diagnosis and prompt treatment are the most effective means of increasing survival rates in cases of oral cancer. Oral cancer is regarded as an easily diagnosed disease because the oral region is an anatomically exposed area, making it easy to examine by direct vision and palpation, as well as to biopsy16. However, diagnosis of oral cancer is usually limited to clinicians: it is unusual for patients to recognize oral tumors. Indeed, we previously reported that patients able to recognize oral tumors by themselves and who later visited the hospital already had an advanced-stage of oral cancer1314, which we hypothesize is the result of the common characteristics of oral cancer, which includes painless insidious growth and symptoms resembling other those of inflammatory oral diseases such as swelling, easy bleeding, and pus discharge. Especially, painless insidious growth results in patients recognizing their disease at a relatively late point, and the similarity of symptoms with other inflammatory oral diseases often leads patients to ignore their symptoms. Ultimately, patients present to the hospital after developing severe symptoms such as discomfort during mastication, deglutition, or breathing or development of facial deformities, and at the time of the interview, patients often report that they did not consider the possibility that their condition was a cancer1718.
The goals of treating oral cancer are complete removal of the tumor while preserving oral function and structure, reduce the frequency of complications and recurrence after treatment, and prevent secondary cancer. To achieve these goals, various treatment methods have been investigated, including surgical excision, radiotherapy, chemotherapy, and immunotherapy. Surgical treatment is the preferred method for radical treatment, and neck dissection can be performed simultaneously when there is a suspicion of neck lymph node metastasis. In cases of early stage disease where there is no neck metastasis, a single-modality surgical treatment or radiotherapy is appropriate. On the other hand, various combination therapies such as surgery and radiotherapy or surgery and chemotherapy have been investigated in advanced stage disease with the goal of improving 5-year survival rates919.
In this study, single surgery (38.1%) and PORT (39.2%) were the most common treatment modalities between 1982–1996. On the other hand, we noted that the treatment methods had diversified by 2007–2011. Specifically, the use of PORT was reduced (20.8%) while use of radiotherapy alone (10.0%), chemotherapy alone (10.0%), and CCRT alone (16.7%) increased. In comparing these time periods according to cancer stage, there were two main treatment methods. Specifically, surgery alone (64.7%) and PORT (53.3%) were the most common methods between 1982–1996, whereas the rate of surgery alone (72.7%) had increased by 2007–2011, PORT (9.1%) decreased, and the use of either chemotherapy or radiotherapy was relatively elevated. As described in the introduction, complete resection of the lesion had the best results in early-stage oral cancer. In stage IV disease, surgery alone (28.6%) and PORT (32.6%) were the most common treatment methods between 1982–1996. However, by 2007–2011, the use of these methods had decreased while the prevalence of the other methods increased in order to compensate, especially CCRT, the use of which increased from 10.2% to 24.1%. In advanced oral cancer, there was no significant difference between chemotherapy and/or radiotherapy, and surgery in terms of 5-year survival rates. However, functional and aesthetic defects that result from radical surgery can reduce a patient's quality of life. Therefore, conservative methods are preferred over radical treatment in older patients.
Because the oral cavity is surrounded primarily by muscles and mucosa, anatomical barriers blocking infiltration of cancer are lacking, and the frequency of local infiltration and cervical lymph node metastasis is correspondingly high. For these reasons, neck surgery for lymph node management is very important. RND was first described by Crile7 and later modified and standardized by Martin et al.20. Classical RND is associated with significant complications, such as increased intracranial blood pressure, decreased range of shoulder abduction, and neck deformity2122. FND and SND are new methods that have been utilized to decrease complications while maintaining lymph node management.
Based on our evaluation of 1982–1996, RND was the most common surgical method (45.5%) and 27.8% of patients received no treatment. Likewise, SND (16.5%) and FND (10.2%) were performed at relatively low frequencies. Conversely, between 2007–2011, only 9.2% of patients either did not undergo surgery or were treated by RND, and the use of SND increased substantially to 57.8%, while FND was also increased to 22.4%. Based on these changes, we determined that the idea of preventative, functional, and conservative neck dissection that preserves vital structures had been applied within our department. This change had occurred alongside the accumulation of experience and advancements regarding oral cancer treatments232425. Many studies have reported no significant difference in 5-year survival rates associated with SND/FND and RND. Conversely, there have been reports that SND or FND are associated with lower mortality compared to RND. Thus, we considered the possibility that the use of classical RND had specifically decreased due to its association with severe complication and/or side-effects, resulting in increased use of conservative neck dissection.
We analyzed 5-year survival rates in order to evaluate the curative effects of different treatments for oral cancer. We confirmed that 12 of 38 patients (31.6%) survived during 1982–199013. Using life table methods, the 5-year survival rate during 1991–1999 was 54%14. In addition, we found that the 5-year survival rates for 1999–2006 and 2007–2011 were 57.7% and 63.5% according to Kaplan-Meier analysis, respectively. In this study, we were unable to evaluate treatment modalities between 1982–1999 and choice of neck dissection methods between 1999–2006 as a result of patient charts having been missed or discarded during our transition to electronic medical records. To address this gap, we utilized results from previous studies, which was sufficient to understand changes in treatment trends and survival rates. Specifically, the National Cancer Institute reported that the 5-year survival rate of oral cancer during 2003–2009 was 62.2%26. Likewise, the Cancer Research UK reported that the 5-year survival rate for oral cancer between 1996–1999 was 50%16. Together, these findings support the idea that our treatment level and 5-year survival rate for oral cancer had caught up with those of developed countries.
In recent years the importance of not only increased survival in patients with oral cancer, but also aesthetic, functional preservation, and social recovery, has become increasingly clear. This shift has resulted in the development of conservative therapies such as chemotherapy, radiotherapy, and CCRT. Moreover, reconstruction though various free-flap procedures can often satisfy patients' aesthetic and functional needs. As a result, our department has undergone large changes in treatment modality, which has been associated with increased 5-year survival rates.
Looking into the future, while the treatment methods for oral cancer in highly developed countries continue to develop, 5-year survival rates have not increased as expected. Indeed, the previously rising 5-year survival rates in developed countries are now beginning to exhibit stagnation, a phenomenon that is especially apparent in advanced oral cancer27. Thus, we can assume that our 5-year survival rates will also be subject to a similar situation. Contributing to this problem is the fact that patients tend to only visit the hospital at relatively late stages of disease due to ignorance regarding oral cancer, the difficulty of establishing a differential diagnosis between pre-cancerous lesions and other oral diseases, misdiagnoses by clinicians, and the economic burdens of treatment. All of these situations result in poorer prognoses for patients. Therefore, the prevention of oral cancer, earlier diagnosis, and active treatment of early stage disease may be the best means of improving 5-year survival rates and quality of life after treatment. Achieving these goals may require the enforcement of public education, and social efforts relevant to early diagnosis through regular oral examinations by expert clinicians.
Over the last 30 years the use of radical surgical treatment has gradually decreased while the prevalence of functional and conservative surgery has increased. Indeed, the use of CCRT has increased remarkably alongside conservative surgery. We found that the 5-year survival rate of patients at our department caught up to the level observed in developed countries over the course of the present study, although most patients continued to present to the hospital with stage IV disease. In the future, prevention, early diagnosis, and active treatment of early stage disease may be the best way of increasing 5-year survival rates.
References
1. Strouhal E. Ancient Egyptian case of carcinoma. Bull N Y Acad Med. 1978; 54:290–302. PMID: 343851.
2. Archer WH, William TG. Morton, dentist, who first publicly demonstrated ether anesthesia; a short biography. J Am Dent Assoc. 1946; 33:1528–1532. PMID: 20273724.
3. Kaplan HS. Historic milestones in radiobiology and radiation therapy. Semin Oncol. 1979; 6:479–489. PMID: 119321.
4. Buschke F. Radiation therapy. Historical perspectives. Radiol Clin Biol. 1971; 40:217–220. PMID: 4938754.
5. Cade S. The radium treatment of cancer of the tongue. Can Med Assoc J. 1930; 23:771–774. PMID: 20318081.
6. Goodman LS, Wintrobe MM, Dameshek W, Goodman MJ, Gilman A, McLennan MT. Nitrogen mustard therapy; use of methyl-bis (beta-chloroethyl) amine hydrochloride and tris (beta-chloroethyl) amine hydrochloride for Hodgkin's disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. J Am Med Assoc. 1946; 132:126–132. PMID: 20997191.
7. Crile G. Excision of cancer of the head and neck: with special reference to the plan of dissection based on one hundred and thirtytwo operations. JAMA. 1906; 47:1780–1786.
8. Butlin HT. An address on the results of operations for carcinoma of the tongue, with an analysis of 197 cases: delivered before the International Surgical Society in Brussels, September, 1908. Br Med J. 1909; 1:1.b2–1.b6. PMID: 20764214.

9. Shah JP, Gil Z. Current concepts in management of oral cancer: surgery. Oral Oncol. 2009; 45:394–401. PMID: 18674952.
10. Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002; 52:23–47. PMID: 11814064.

11. SEER Data, 1973-2012 [Internet]. Bethesda (MD): NIH National Cancer Institute; Surveillance, Epidemiology, an End Results Program;cited 2011 Aug 1. Available from: http://seer.cancer.gov/data/.
12. Cancer survival: England and Wales, less common cancers by age group [Internet]. London: Office for National Statistics (ONS);2005. 3. 02. cited 2011 Aug 1. Available from: http://www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-252716.
13. Cho JH, Kim CS. Clinical and statistical analysis of the oral cancer patients: a statistical analysis of 256 cases. J Korean Assoc Maxillofac Plast Reconstr Surg. 1998; 20:33–44.
14. Kim MY, Kim CS, Lee SH, Kim JW, Jang HJ. A clinicostatistical analysis of oral cancer patients for recent 8 years. J Korean Assoc Oral Maxillofac Surg. 2007; 33:660–668.
15. Cho YS. A clinical study on squamous cell carcinoma of the oral cavity of Korean. J Korean Assoc Oral Maxillofac Surg. 1992; 18:40–52.
16. Hoffman HT, Karnell LH, Funk GF, Robinson RA, Menck HR. The National Cancer Data Base report on cancer of the head and neck. Arch Otolaryngol Head Neck Surg. 1998; 124:951–962. PMID: 9738803.

17. Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008; 44:10–22. PMID: 17825602.

18. Noonan B. Understanding the reasons why patients delay seeking treatment for oral cancer symptoms from a primary health care professional: an integrative literature review. Eur J Oncol Nurs. 2014; 18:118–124. PMID: 24012186.

19. Shah JP, Lydiatt W. Treatment of cancer of the head and neck. CA Cancer J Clin. 1995; 45:352–368. PMID: 7583907.

20. Martin H, Del Valle B, Ehrlich H, Cahan WG. Neck dissection. Cancer. 1951; 4:441–499. PMID: 14839606.

21. Nahum AM, Mullally W, Marmor L. A syndrome resulting from radical neck dissection. Arch Otolaryngol. 1961; 74:424–428. PMID: 14477989.

22. Fitz-Hugh GS, Robins RB, Craddock WD. Increased intracranial pressure complicating unilateral neck dissection. Laryngoscope. 1966; 76:893–906. PMID: 5949296.

23. Medina JE, Byers RM. Supraomohyoid neck dissection: rationale, indications, and surgical technique. Head Neck. 1989; 11:111–122. PMID: 2722487.

24. Byers RM. Modified neck dissection: a study of 967 cases from 1970 to 1980. Am J Surg. 1985; 150:414–421. PMID: 4051103.
25. Byers RM, Wolf PF, Ballantyne AJ. Rationale for elective modified neck dissection. Head Neck Surg. 1988; 10:160–167. PMID: 3235344.

27. Pulte D, Brenner H. Changes in survival in head and neck cancers in the late 20th and early 21st century: a period analysis. Oncologist. 2010; 15:994–1001. PMID: 20798198.

Fig. 2
The 5-year survival rate during 2007–2011 (Kaplan-Meier survival curve). A. Total 5-year survival rates. B. Stratified by tumor stage.
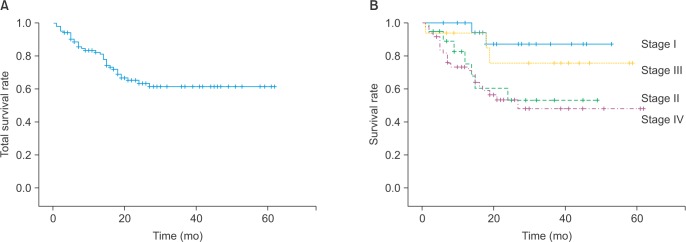
Table 1
Treatment modalities according to tumor stage for patients with oral cancer
| Treatment modality | Stage | Total (%) | ||||
|---|---|---|---|---|---|---|
| Undefined | I | II | III | IV | ||
| 1982-199613 (n=97) | ||||||
| S only | 2 | 7 | 7 | 7 | 14 | 37 (38.1) |
| RT only | - | - | - | - | 8 | 8 (8.2) |
| CT only | - | - | - | - | 3 | 3 (3.1) |
| S+RT | - | 8 | 8 | 6 | 16 | 38 (39.2) |
| CT & RT | 1 | - | 1 | - | 5 | 7 (7.2) |
| S+CT | - | - | 1 | - | 1 | 2 (2.1) |
| S+CT & RT | - | - | - | - | 1 | 1 (1.0) |
| CT & RT+S | - | - | - | - | 1 | 1 (1.0) |
| Total (%) | 3 (3.1) | 15 (15.5) | 17 (17.5) | 13 (13.4) | 49 (50.5) | 97 (100) |
| 2007-2011 (n=120) | ||||||
| S only | 1 | 16 | 12 | 6 | 9 | 44 (36.7) |
| RT only | 1 | 1 | 2 | - | 8 | 12 (10) |
| CT only | 1 | 2 | 2 | 2 | 5 | 12 (10) |
| S+RT | 1 | 2 | 3 | 5 | 14 | 25 (20.8) |
| CT & RT | 1 | 1 | 1 | 4 | 13 | 20 (16.7) |
| S+CT | - | - | - | 1 | - | 1 (0.8) |
| S+CT & RT | - | - | 1 | - | 3 | 4 (3.3) |
| CT & RT+S | - | - | - | - | 2 | 3 (2.5) |
| Total (%) | 5 (4.2) | 22 (18.3) | 21 (17.5) | 18 (15) | 54 (45) | 120 (100) |
(S: surgery, RT: radiation therapy, CT: chemotherapy, +: sequentially, &: synchronously)
Data from the article of Cho and Kim (J Korean Assoc Maxillofac Plast Reconstr Surg 1998;20:33–44)13.
Table 2
Neck dissection in patients with oral cancer according to tumor stage
| Treatment modality | Stage | Total (%) | ||||
|---|---|---|---|---|---|---|
| Undefined | I | II | III | IV | ||
| 1982-199613 (n=79) | ||||||
| No ND | - | 7 | 2 | 3 | 10 | 22 (27.8) |
| Ip. RND | - | - | 7 | 10 | 17 | 34 (43.0) |
| Ip. SND | - | 7 | 2 | - | 3 | 12 (15.2) |
| Ip. FND | - | 1 | 5 | - | 1 | 7 (8.9) |
| Bi. SND | - | - | - | - | 1 | 1 (1.3) |
| Bi. FND | - | - | - | - | 1 | 1 (1.3) |
| Ip. RND+Con. FND | - | - | - | - | 2 | 2 (2.5) |
| Total (%) | - | 15 (9.0) | 16 (10.0) | 13 (8.5) | 35 (43.5) | 79 (100) |
| 2007-2011 (n=76) | ||||||
| No ND | - | 4 | 1 | 1 | 1 | 7 (9.2) |
| Ip. RND | 1 | - | 2 | 1 | - | 4 (5.3) |
| Ip. SND | 1 | 11 | 8 | 5 | 16 | 41 (53.9) |
| Ip. FND | - | 3 | 3 | 3 | 8 | 17 (22.4) |
| Bi. SND | - | - | 2 | - | 1 | 3 (3.9) |
| Ip. FND+Con. SND | - | - | - | - | 1 | 1 (1.3) |
| Ip. RND+Con. FND | - | - | - | 2 | 1 | 3 (3.9) |
| Total (%) | 2 (2.6) | 18 (23.7) | 16 (21.1) | 12 (15.8) | 28 (36.8) | 76 (100) |
(ND: neck dissection, Ip.: ipsilateral, RND: radical neck dissection, SND: supraomohyoid neck dissection, FND: functional neck dissection, Bi.: bilateral, +: sequentially, Con.: contralateral)
Data from the article of Cho and Kim (J Korean Assoc Maxillofac Plast Reconstr Surg 1998;20:33–44)13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download