Abstract
We report a case of retiform hemangioendothelioma (RH) located in the infratemporal fossa and buccal area in a 13-year-old Korean boy. The tumor originated from the sphenoid bone of the infratemporal fossa area and spread into the cavernous sinus, orbital apex, and retro-nasal area with bone destruction of the pterygoid process. Tumor resection was conducted via Le Fort I osteotomy and partial maxillectomy to approach the infratemporal fossa and retro-nasal area. The diagnosis of RH was confirmed after surgery. In the presented patient, surgical excision was incomplete, and close follow-up was performed. There was no evidence of expansion or metastasis of the residual tumor in the 8 years after surgery. In cases of residual RH with low likelihood of expansion and metastasis, even though RH is an intermediate malignancy, close follow-up can be the appropriate treatment choice over additional aggressive therapy. To date, 29 papers and 48 RH cases have been reported, including this case. This case is the second reported RH case presenting as primary bone tumor and the first case originating in the oromaxillofacial area.
Retiform hemangioendothelioma (RH) was first described as a new entity by Calonje et al.1 in 1994. It is considered a rare vascular neoplasm of intermediate stage between benign hemangioma and malignant angiosarcoma, with a high rate of local recurrence and a low rate of metastasis. Histologically, RH presents as an irregular pattern of elongated and arborising vessels like a normal rete testis. Neoplastic vessels are lined by a single layer of hobnail-like endothelial cells that protrude within the narrow lumina1. These endothelial cells usually extend to the deep dermis or subcutaneous tissues2.
To date, 29 papers and 48 RH cases have been reported, including the present case.(Table 1)12345678910111213141516171819202122232425262728 Lesions were located in the upper and lower extremities in 32 cases, the trunk (including one case of penile location) in 10 cases, and the head and neck area in 7 cases. Most RHs originate from vessels at the superficial portion of the body including dermal or subcutaneous tissues. However, Aditya et al.3 reported a case of RH presenting as a primary bone tumor originating from the parietal bone.
In our patient, the symptom of epistaxis was followed by identification of a buccal mass. A heterogeneous mass in the infratemporal fossa and bone destruction of the pterygoid process were observed on magnetic resonance imaging (MRI). (Fig. 1, 2) Tumor resection was conservatively conducted by Le Fort I osteotomy and a posterior partial maxillectomy following incisional biopsy demonstrating a juvenile capillary hemangioma. Portions of the tumor remained in the pterygopalatine fossa, the inferior orbital fissure, and the anterior cavernous sinus. The final pathological report confirmed RH in the infratemporal fossa. Despite tumor remnants, no evidence of extension or metastasis was detected for 8 years of follow-up. The origin was inferred to be the sphenoid bone in the infratemporal fossa area. This is the second RH case of primary bone tumor and the first case of oromaxillofacial area origin.
A 13-year-old Korean boy presented with frequent epistaxis and right buccal cheek swelling. Frequent epistaxis had occurred for the previous year. Buccal swelling was first recognized one month prior. Clinically, a solid mass was palpated on the buccal area, and there was no pain or paresthesia.(Fig. 1) The patient also complained of loss of appetite, fatigue, and dizziness. Blood test revealed iron deficiency anemia status of red blood cell 2.68×106/µL, hemoglobin 5.0 g/dL, mean corpuscular volume 65 fL, mean corpuscular hemoglobin 18.7 pg, and mean corpuscular hemoglobin concentration 29 g/dL. Continuous nasal bleeding was suspected to be the main reason for anemia.
MRI showed a well-defined mass in the right infratemporal fossa and buccal area with extension to the intracranial cavernous sinus through the foramen rotundum, the orbital apex through the inferior orbital fissure, and the retro-nasal area, with bone destruction of the pterygoid process. The posterior wall of the right maxillary sinus was remodeled. T2-weighted images revealed heterogeneous low-signal area and intermediate to high signal intensity in the nasal cavity. T1-weighted images showed intermediate to low signal intensity similar to that of muscles and relatively homogeneous patterns.(Fig. 2) Tumor size was approximately 5.0×3.0×2.0 cm. Local lymph node or distant metastasis was not observed.
Intraoral incisional biopsy of the buccal cheek area was initially performed and diagnosed as juvenile capillary hemangioma. Complete excision was recommended to rule out mixed hemangioma, composite hemangioma, or hemangioendothelioma.
A Le Fort I osteotomy was performed, and the maxilla was retracted downward. This approach revealed relatively well-defined masses in the infratemporal fossa and retro-nasal area. We initially dissected these masses in the infratemporal fossa, and conducted posterior partial maxillectomy including bethe destructed pterygoid process and the remodeled portion of the maxillary posterior wall and tuberosity. Tumor masses adjacent to the inferior orbital fissure and retro-nasal area were segmentally dissected.(Fig. 3) Anatomically, the complete removal of masses in the deep portion of the infraorbital fissure and cavernous sinus was not possible. Loss of vision and neurological deficits are critical complications that cannot be risked.
Histological examination of the tumor revealed an irregular pattern of elongated and arborising vessels. Neoplastic vessels with hyaline core were lined by a single layer of hobnaillike endothelial cells that protruded within the narrow lumina. Dense sclerotic intervening stroma and focal lymphocytes infiltrated around vessels. Immunohistochemical stain results were positive for CD31, CD34, and factor VIII-related antigen in endothelial cells.(Fig. 4) Vascular wall showed weak patchy positivity for smooth muscle actin (SMA) and desmin. Positivity for Ki-67 was less than 0.1%. The final histological diagnosis was RH.
Epistaxis subsided after surgery, and anemia status was resolved to the normal range. Postoperative MRI showed a small enhancing soft tissue lesion in the pterygopalatine fossa, inferior orbital fissure, and anterior cavernous sinus, confirming residual tumor segments. No significant interval changes were observed on follow-up MRI over 4 years (Fig. 5), and no clinical neurological signs or symptoms were seen over 8 years of follow-up except for facial asymmetry due to under-development on the right face.
RH is a rare vascular neoplasm of intermediate stage beintween benign hemangioma and malignant angiosarcoma with a high rate of local recurrence and low rate of metastasis1. Most reported cases originate from cutaneous or superficial portions of the body, except for one case of primary bone tumor reported by Aditya et al.3. The tumor has nonspecific clinical and radiological features, but this infiltrative neoplasm is histologically characterized by elongated arborizing vessels, arranged in an anastomosing pattern that resembles the normal rete testis, and is lined with a single layer of matchstick or hobnail-like endothelial cells that protrude within the narrow lumina4.
Tan et al.5 in 2005 reviewed 24 cases of RH, and the number of reported cases has doubled within one decade. To date, 29 papers and 48 RH cases have been reported, including this case.(Table 1)12345678910111213141516171819202122232425262728 There has been a female predominance (female:male, 32:16), and the patients were predominantly young and middle-aged adults in 2005. However, in this review, patients under 20 years represented nearly 30% of the sample, and patients over 50 years accounted for nearly 20%. In addition, Serel et al.6 reported a congenital RH case. This means that the remarkable predominant generation is not defined. Most tumor sizes were less than 10 cm except in two cases. The mean duration before treatment was approximately three years. This data implies that RH grows slowly and asymptomatically at the beginning.
RH location was a limb in 32 cases (65.3%), the trunk including one case of penis in 10 cases (20.4%), and head and neck in 7 cases (14.3%). Among RHs in the head and neck area, five originated from the superficial portion of body including scalp, canthus, or pinna. One case originated from the parietal bone. Most other RHs originated from vessels in a superficial area of the body like skin or subcutaneous tissue. RH cells usually extend to the deep dermis or subcutaneous tissues1. However, three cases including this case showed bone-involved lesions. O'Duffy et al.7 presented an active mass involving the left pinna with signs of left mastoid osseous involvement, but the origin was the pinna. Aditya et al.3 first reported RH as a primary bone tumor; computed tomography (CT) images showed destruction of the parietal bone, and the skin over it was essentially normal. The present case is the second of RH presenting as primary bone tumor and the first originating from the oromaxillofacial area. The lesion started from the sphenoid bone of the infratemporal fossa and extended to the buccal area, orbital apex, intracranial cavernous sinus, and retro-nasal area. These two cases imply no limitation of the origin of RH other than high anatomic vascularity.
Half of RH locally recurred within a mean recurrence period of approximately 12 months after surgery. Nearly half of recurred tumors were detected within six months. Metastasis is rare, only seen in three patients. One case of metastasis was lost to follow-up. In two cases with lymph node metastasis reported by Calonje et al.1 and O'Duffy et al.7, surgical excision with radiation therapy was performed, and the patients remained tumor-free. Metastasis itself does not seem like a fatal prognostic factor.
Two patients died but not due to metastasis. One patient experienced post-surgical hypovolemic shock in the context of diffuse lymphangiomatosis with pulmonary localization8, and the other case, described as aggressive RH of the scalp9, was probably better interpreted as classic angiosarcoma with a focal retiform pattern29.
Histopathological examinations must be conducted for confirmation of diagnosis because RH does not have unique clinical or radiological features. Histopathologically, tumor-involved tissues are infiltrated by vessels like a normal rete testis. Neoplastic vessels with hyaline core are lined with a single layer of matchstick or hobnail-like endothelial cells. Dense sclerotic intervening stroma and focal lymphocytes infiltrate around vessels. Two features of absence of dissection between individual collagen bundles and absence of endothelial atypia or mitotic activity distinguish RH from angiosarcoma. This low-grade neoplasm recurs frequently but has a very low metastatic rate1. Immunohistochemical stain results were positive for CD31, CD34, and factor VIII-related antigen in neoplastic endothelial cells, and the vascular wall was negative for SMA29. These previously identified histological findings were consistent with our results. The vascular wall showed weak patchy positivity for SMA, and desmin Ki-67 positivity was less than 0.1% in this case. Unfortunately, most final diagnoses of previous RHs were confirmed after surgery. Preoperative biopsy helps with diagnosis but is not always available. For this reason, postoperative management after confirmed RH diagnosis should be carefully considered with surgical treatment.
Requena and Kutzner29 summarized hemangioendotheliomas (HE) and presented seven subtypes including RH, spindle cell HE (hemangioma), papillary intralymphatic angioendothelioma (Dabska tumor), RH, kaposiform HE, epithelioid HE, pseudomyogenic HE (epithelioid sarcoma-like HE), and composite HE9. These vascular neoplasms have borderline biological behavior, an intermediate stage between entirely benign hemangiomas and highly malignant angiosarcoma. All cases of spindle cell HE showed benign biological behavior and so were categorized as hemangioma. Epithelioid HE appears to have more aggressive biological behavior than the other subtypes. The other five subtypes including RH are truly intermediate stage. Even though RH locally recurs with a high rate, no deaths have been reported.
The need for surgical treatment and additional postoperative therapy for RH should be decided in consideration of this intermediate character. Radiotherapy was conducted in six patients. Five were successfully treated, and one patient died but the diagnosis was considered classic angiosarcoma9. In addition, Hirsh et al.10 first reported successful treatment of RH with low-dose cisplatin and moderate radiotherapy without surgical resection. Use of surgical treatment and adjuvant therapies should be carefully considered to avoid unnecessary interventions and potential severe complications.
For the approach to the infratemporal area and the lateral skull base, the infratemporal fossa approach has been commonly used30. However, this route has complications of hearing loss, facial nerve palsy, and scar caused by a periauricular incision. The approach to the nasal cavity is also limited. Roche et al.31 proposed surgery of the infratemporal fossa using the minimally invasive transmaxillary approach. The route is convenient for exposing the structures of the anterior part of the infratemporal fossa including retro-nasal area, pterygomaxillary fissure, and pterygopalatine fossa. Le Fort I osteotomy is nearly the same as the transmaxillary approach, which is familiar to oral and maxillofacial surgeons.
In our case, considering the preoperative diagnosis of juvenile capillary hemangioma and the intraoperatively benign character, the tumor mass was conservatively excised around the cavernous sinus and orbital apex. Le Fort I osteotomy with posterior partial maxillectomy was conducted to simultaneously approach the infratemporal fossa, buccal, retronasal, and posterior maxilla area. The patient's epistaxis subsided after surgery. However, postoperative CT images showed small enhancing soft tissue lesions in the pterygopalatine fossa, inferior orbital fissure, and anterior cavernous sinus, confirming residual tumor segments. As discussed above, given the intermediate tendency of RH, we decided not to perform further procedures. Close follow-up was performed. There was no evidence of expansion or metastasis during follow-up with MRI over 4 years and no clinical signs or symptoms over 8 years of follow-up.
In conclusion, RHs originate not only from the superficial vessels of the body, but also from deep structures such as the sphenoid bone of the infratemporal fossa area that has anatomically high vascularity. Half of all reported recurrences presented within six months, and close follow-up is recommended during this period. If the residual RH has relatively benign tendency and does not expand or metastasize, close follow-up can be the appropriate treatment choice rather than additional aggressive therapy.
References
1. Calonje E, Fletcher CD, Wilson-Jones E, Rosai J. Retiform hemangioendothelioma. A distinctive form of low-grade angiosarcoma delineated in a series of 15 cases. Am J Surg Pathol. 1994; 18:115–125. PMID: 8291650.
2. Fukunaga M, Endo Y, Masui F, Yoshikawa T, Ishikawa E, Ushigome S. Retiform haemangioendothelioma. Virchows Arch. 1996; 428:301–304. PMID: 8764941.

3. Aditya GS, Santosh V, Yasha TC, Shankar SK. Epithelioid and retiform hemangioendothelioma of the skull bone--report of four cases. Indian J Pathol Microbiol. 2003; 46:645–649. PMID: 15025366.
4. El Darouti M, Marzouk SA, Sobhi RM, Bassiouni DA. Retiform hemangioendothelioma. Int J Dermatol. 2000; 39:365–368. PMID: 10849129.

5. Tan D, Kraybill W, Cheney RT, Khoury T. Retiform hemangioendothelioma: a case report and review of the literature. J Cutan Pathol. 2005; 32:634–637. PMID: 16176302.

6. Serel S, Serel BI, Uluc A, Heper AO, Gultan MS. Congenital retiform hemangioendothelioma. Indian J Dermatol. 2007; 52:160–162.

7. O'Duffy F, Timon C, Toner M. A rare angiosarcoma: retiform haemangioendothelioma. J Laryngol Otol. 2012; 126:200–202. PMID: 21888747.
8. Albertini AF, Brousse N, Bodemer C, Calonje E, Fraitag S. Retiform hemangioendothelioma developed on the site of an earlier cystic lymphangioma in a six-year-old girl. Am J Dermatopathol. 2011; 33:e84–e87. PMID: 21915027.

9. Zhang G, Lu Q, Yin H, Wen H, Su Y, Li D, et al. A case of retiform-hemangioendothelioma with unusual presentation and aggressive clinical features. Int J Clin Exp Pathol. 2010; 3:528–533. PMID: 20606734.
10. Hirsh AZ, Yan W, Wei L, Wernicke AG, Parashar B. Unresectable retiform hemangioendothelioma treated with external beam radiation therapy and chemotherapy: a case report and review of the literature. Sarcoma. 2010; DOI: 10.1155/2010/756246.

11. Duke D, Dvorak A, Harris TJ, Cohen LM. Multiple retiform hemangioendotheliomas. A low-grade angiosarcoma. Am J Dermatopathol. 1996; 18:606–610. PMID: 8989934.
12. Dufau JP, Pierre C, De Saint Maur PP, Bellavoir A, Gros P. Retiform hemangioendothelioma. Ann Pathol. 1997; 17:47–51. PMID: 9162159.
13. Mentzel T, Stengel B, Katenkamp D. Retiform hemangioendothelioma. Clinico-pathologic case report and discussion of the group of low malignancy vascular tumors. Pathologe. 1997; 18:390–394. PMID: 9432675.
14. Sanz-Trelles A, Rodrigo-Fernandez I, Ayala-Carbonero A, Contreras-Rubio F. Retiform hemangioendothelioma. A new case in a child with diffuse endovascular papillary endothelial proliferation. J Cutan Pathol. 1997; 24:440–444. PMID: 9274963.

15. Schommer M, Herbst RA, Brodersen JP, Kiehl P, Katenkamp D, Kapp A, et al. Retiform hemangioendothelioma: another tumor associated with human herpesvirus type 8? J Am Acad Dermatol. 2000; 42:290–292. PMID: 10642690.

16. Ulrich D, Hrynyschyn K. Fast growing multifocal retiform hemangioendothelioma: a case report. Eur J Plast Surg. 2002; 25:47–49.
17. Coutinho MLF. Laser indications hemangiomas in children. An Acad Nac Med. 2002; 162:74–76.
18. Escudero AG, Sanchez JS, Bustos GN, Serrano TG, Martin JJR, Campora RG. Retiform hemangioendothelioma: a report of two cases and literature review. Acta Dermosifiliogr. 2003; 94:102–106.
19. Botros MF, Abdo I, Foda S, Amer H, Attia N, Saad S, et al. Retiform hemangioendothelioma. Egypt Dermatol Online J. 2005; 1:1–4.
20. Ioannidou D, Panayiotides J, Krasagakis K, Stefanidou M, Manios A, Tosca A. Retiform hemangioendothelioma presenting as bruiselike plaque in an adult woman. Int J Dermatol. 2006; 45:53–55. PMID: 16426378.

21. Parsons A, Sheehan DJ, Sangueza OP. Retiform hemangioendotheliomas usually do not express D2-40 and VEGFR-3. Am J Dermatopathol. 2008; 30:31–33. PMID: 18212541.

22. Love WE, Keiler SA, Tamburro JE, Honda K, Gosain AK, Bordeaux JS. Surgical management of congenital dermatofibrosarcoma protuberans. J Am Acad Dermatol. 2009; 61:1014–1023. PMID: 19925926.

23. Bhutoria B, Konar A, Chakrabarti S, Das S. Retiform hemangioendothelioma with lymph node metastasis: a rare entity. Indian J Dermatol Venereol Leprol. 2009; 75:60–62. PMID: 19172034.

24. Emberger M, Laimer M, Steiner H, Zelger B. Retiform hemangioendothelioma: presentation of a case expressing D2-40. J Cutan Pathol. 2009; 36:987–990. PMID: 19674202.

25. Kajo K, Macháleková K, Pauer M. Retiform hemangioendotelioma in a 8-year-old girl--case report. Cesk Patol. 2009; 45:72–74. PMID: 19764161.
26. Aydingöz IE, Mansur AT, Celasun B. Correspondence: retiform hemangioendothelioma presenting as a hyperhidrotic tumor. Int J Dermatol. 2010; 49:1076–1077. PMID: 20883275.
27. Choi WK, Lee SH, Oh SA, Kang DH. Retiform hemangioendothelioma on the finger. Arch Plast Surg. 2012; 39:80–82. PMID: 22783501.

28. Findikçioğlu K, Findikçioğlu F, Kutlugün C, Karabağli P. Retiform hemangioendothelioma originated from capillary malformation: a rare case. Turk J Plast Surg. 2012; 20:12–15.
30. Zhang M, Garvis W, Linder T, Fisch U. Update on the infratemporal fossa approaches to nasopharyngeal angiofibroma. Laryngoscope. 1998; 108:1717–1723. PMID: 9818832.

31. Roche PH, Fournier HD, Laccourreye L, Mercier P. Surgical anatomy of the infratemporal fossa using the transmaxillary approach. Surg Radiol Anat. 2001; 23:209–213. PMID: 11694963.

Fig. 1
Preoperative clinical photograph. A. Extraoral image of buccal cheek swelling. B. Intraoral image of buccal cheek swelling.
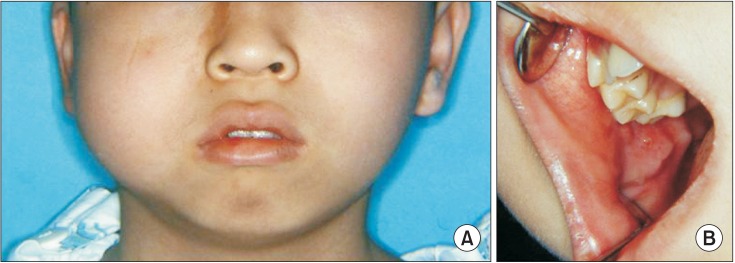
Fig. 2
Preoperation magnetic resonance imaging. A. Axial T2-weighted image of low signal intensity in posterior maxillar and retro-nasal area with bony destruction of pterygoid process (black and white arrows). B. Axial T2-weighted image of homogeneous low signal intensity in buccal area. C. Coronal T2-weighted image of heterogeneous low signal intensity in retro-nasal cavity, infratemporal fossa, and cavernous sinus through foramen rotundum (arrows). D. Sagittal T1-weighted image of homogeneous low signal intensity in infratemporal fossa, and orbital apex through infraorbital fissure (arrows).
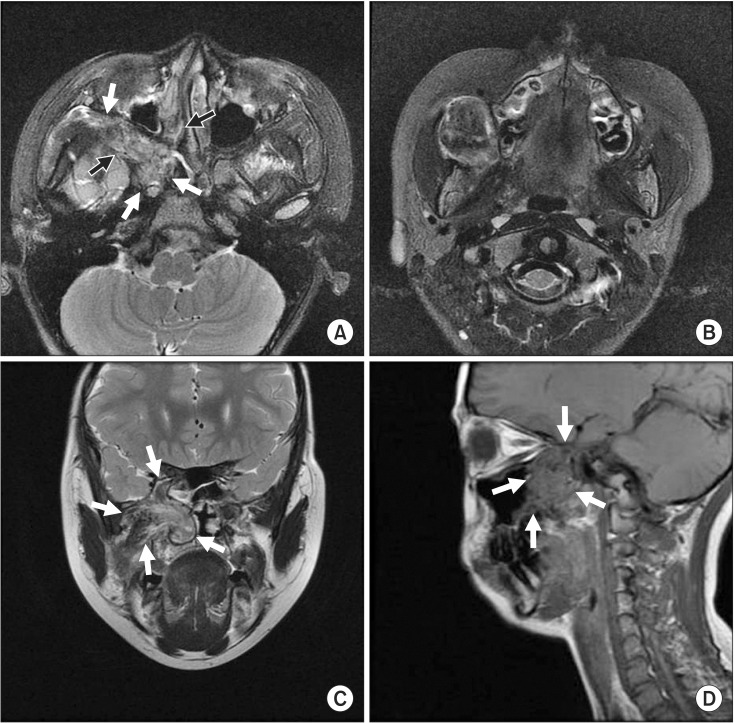
Fig. 3
Intraoperative photograph. A. Le Fort I osteotomy reveals buccal, infratemporal fossa, maxillary sinus and nasal cavity. B. Main mass of buccal, infratemporal and retro-nasal area. C. Segmentally resected masses of inferior orbital fissure area.
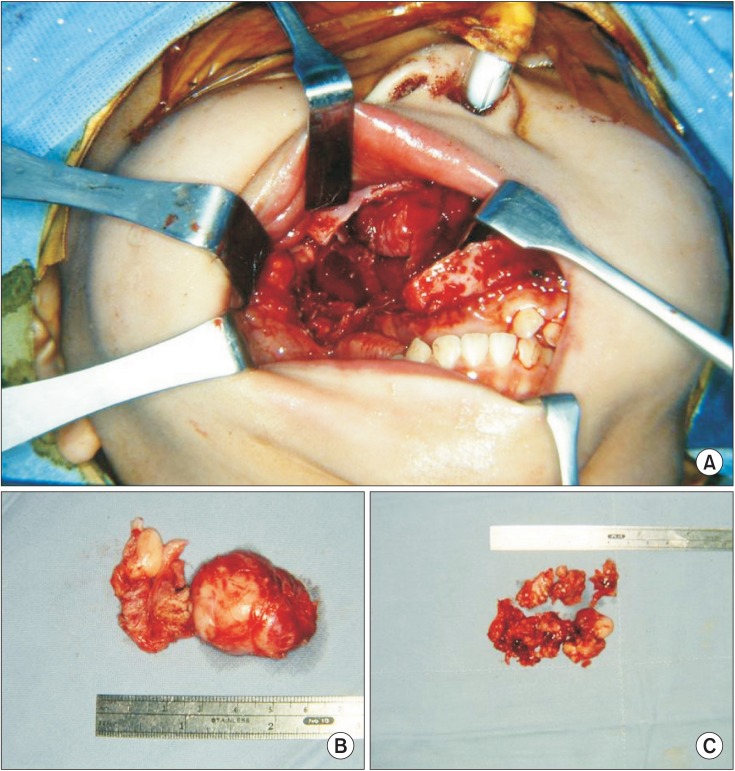
Fig. 4
Microscopic findings of retiform hemangioendothelioma. A. Characteristic elongated and narrow arborizing vascular channels and focal lymphocytic infiltrate (H&E staining, ×40). B. Monomorphic endothelial cells with hobnail appearance simulate the architecture of rete testis (H&E staining, ×200). C. Vacuolated cells and focal papillae with hyaline core (H&E staining, ×200). D. Neoplastic cells are immunohistochemically reactive for vascular marker, CD31 (immunohistochemistry staining, ×200).
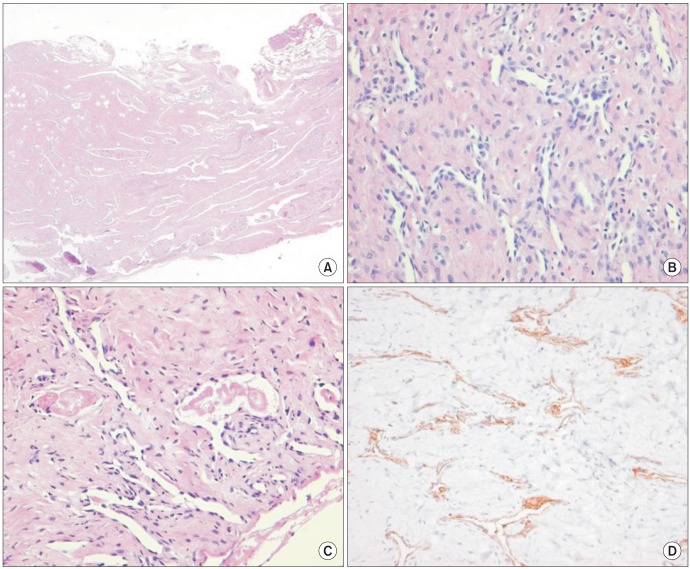
Fig. 5
Seven-month (A, B) and four-year (C, D) follow-up magnetic resonance imaging demonstrating residual tumor segments on infratemporal fossa area with no significant interval changes. A, C. Aixial T2-weighted images of low signal intensity in infratemporal fossa area and inferior orbital fissure (black and white arrows). B, D. Coronal T2-weighted images of low signal intensity in cavernous sinus and pterygoid plate (black and white arrows).
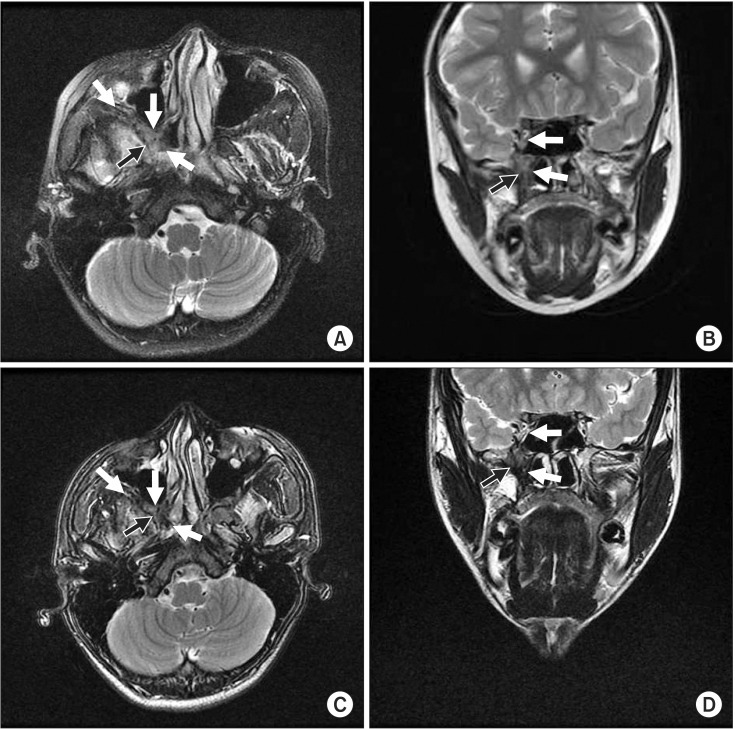
Table 1
Tumor location of 48 reported cases of retiform hemangioendothelioma including the present case
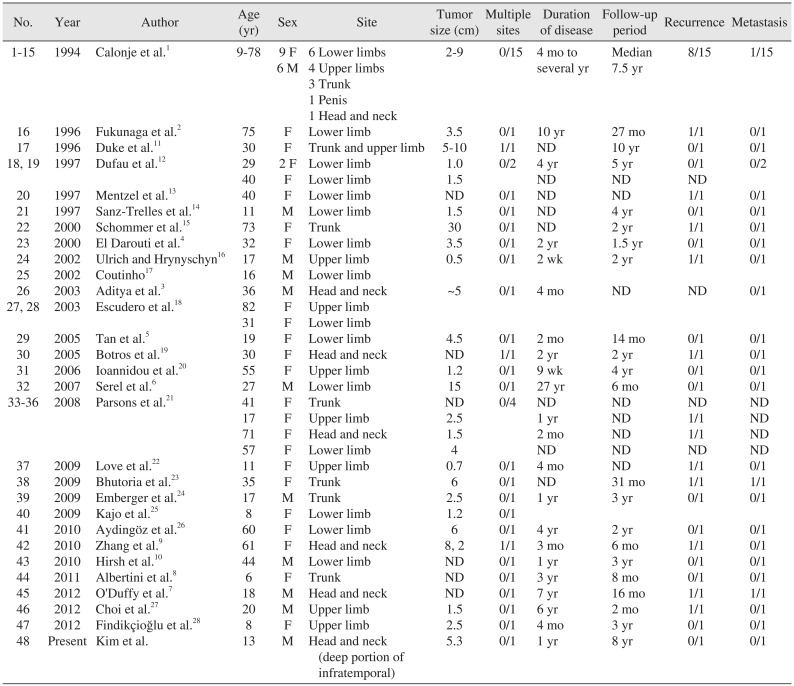
| No. | Year | Author | Age (yr) | Sex | Site | Tumor size (cm) | Multiple sites | Duration of disease | Follow-up period | Recurrence | Metastasis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–15 | 1994 | Calonje et al.1 | 9–78 | 9 F | 6 Lower limbs | 2–9 | 0/15 | 4 mo to several yr | Median 7.5 yr | 8/15 | 1/15 |
| 6 M | 4 Upper limbs | ||||||||||
| 3 Trunk | |||||||||||
| 1 Penis | |||||||||||
| 1 Head and neck | |||||||||||
| 16 | 1996 | Fukunaga et al.2 | 75 | F | Lower limb | 3.5 | 0/1 | 10 yr | 27 mo | 1/1 | 0/1 |
| 17 | 1996 | Duke et al.11 | 30 | F | Trunk and upper limb | 5–10 | 1/1 | ND | 10 yr | 0/1 | 0/1 |
| 18, 19 | 1997 | Dufau et al.12 | 29 | 2 F | Lower limb | 1.0 | 0/2 | 4 yr | 5 yr | 0/1 | 0/2 |
| 40 | F | Lower limb | 1.5 | ND | ND | ND | |||||
| 20 | 1997 | Mentzel et al.13 | 40 | F | Lower limb | ND | 0/1 | ND | ND | 1/1 | 0/1 |
| 21 | 1997 | Sanz-Trelles et al.14 | 11 | M | Lower limb | 1.5 | 0/1 | ND | 4 yr | 0/1 | 0/1 |
| 22 | 2000 | Schommer et al.15 | 73 | F | Trunk | 30 | 0/1 | ND | 2 yr | 1/1 | 0/1 |
| 23 | 2000 | El Darouti et al.4 | 32 | F | Lower limb | 3.5 | 0/1 | 2 yr | 1.5 yr | 0/1 | 0/1 |
| 24 | 2002 | Ulrich and Hrynyschyn16 | 17 | M | Upper limb | 0.5 | 0/1 | 2 wk | 2 yr | 1/1 | 0/1 |
| 25 | 2002 | Coutinho17 | 16 | M | Lower limb | ||||||
| 26 | 2003 | Aditya et al.3 | 36 | M | Head and neck | ~5 | 0/1 | 4 mo | ND | ND | 0/1 |
| 27, 28 | 2003 | Escudero et al.18 | 82 | F | Upper limb | ||||||
| 31 | F | Lower limb | |||||||||
| 29 | 2005 | Tan et al.5 | 19 | F | Lower limb | 4.5 | 0/1 | 2 mo | 14 mo | 0/1 | 0/1 |
| 30 | 2005 | Botros et al.19 | 30 | F | Head and neck | ND | 1/1 | 2 yr | 2 yr | 1/1 | 0/1 |
| 31 | 2006 | Ioannidou et al.20 | 55 | F | Upper limb | 1.2 | 0/1 | 9 wk | 4 yr | 0/1 | 0/1 |
| 32 | 2007 | Serel et al.6 | 27 | M | Lower limb | 15 | 0/1 | 27 yr | 6 mo | 0/1 | 0/1 |
| 33-36 | 2008 | Parsons et al.21 | 41 | F | Trunk | ND | 0/4 | ND | ND | ND | ND |
| 17 | F | Upper limb | 2.5 | 1 yr | ND | 1/1 | ND | ||||
| 71 | F | Head and neck | 1.5 | 2 mo | ND | 1/1 | ND | ||||
| 57 | F | Lower limb | 4 | ND | ND | ND | ND | ||||
| 37 | 2009 | Love et al.22 | 11 | F | Upper limb | 0.7 | 0/1 | 4 mo | ND | 1/1 | 0/1 |
| 38 | 2009 | Bhutoria et al.23 | 35 | F | Trunk | 6 | 0/1 | ND | 31 mo | 1/1 | 1/1 |
| 39 | 2009 | Emberger et al.24 | 17 | M | Trunk | 2.5 | 0/1 | 1 yr | 3 yr | 0/1 | 0/1 |
| 40 | 2009 | Kajo et al.25 | 8 | F | Lower limb | 1.2 | 0/1 | ||||
| 41 | 2010 | Aydingöz et al.26 | 60 | F | Lower limb | 6 | 0/1 | 4 yr | 2 yr | 0/1 | 0/1 |
| 42 | 2010 | Zhang et al.9 | 61 | F | Head and neck | 8, 2 | 1/1 | 3 mo | 6 mo | 1/1 | 0/1 |
| 43 | 2010 | Hirsh et al.10 | 44 | M | Lower limb | ND | 0/1 | 1 yr | 3 yr | 0/1 | 0/1 |
| 44 | 2011 | Albertini et al.8 | 6 | F | Trunk | ND | 0/1 | 3 yr | 8 mo | 0/1 | 0/1 |
| 45 | 2012 | O'Duffy et al.7 | 18 | M | Head and neck | ND | 0/1 | 7 yr | 16 mo | 1/1 | 1/1 |
| 46 | 2012 | Choi et al.27 | 20 | M | Upper limb | 1.5 | 0/1 | 6 yr | 2 mo | 1/1 | 0/1 |
| 47 | 2012 | Findikçioğlu et al.28 | 8 | F | Upper limb | 2.5 | 0/1 | 4 mo | 3 yr | 0/1 | 0/1 |
| 48 | Present | Kim et al. | 13 | M | Head and neck (deep portion of infratemporal) | 5.3 | 0/1 | 1 yr | 8 yr | 0/1 | 0/1 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download