Abstract
Submucosal infiltration and the topical application of epinephrine as a vasoconstrictor produce excellent hemostasis during surgery. The hemodynamic effects of epinephrine have been documented in numerous studies. However, its metabolic effects (especially during surgery) have been seldom recognized clinically. We report two cases of significant metabolic effects (including lactic acidosis and hyperglycemia) as well as hemodynamic effects in healthy patients undergoing orthognathic surgery with general anesthesia. Epinephrine can induce glycolysis and pyruvate generation, which result in lactic acidosis, via β2-adrenergic receptors. Therefore, careful perioperative observation for changes in plasma lactate and glucose levels along with intensive monitoring of vital signs should be carried out when epinephrine is excessively used as a vasoconstrictor during surgery.
Blood loss during orthognathic surgery can be excessive, especially in multiple jaw surgeries. The use of a local anesthetic in conjunction with a vasoconstrictor is the most effective means of reducing blood loss in these procedures. Epinephrine is the most commonly used vasoconstrictor123. This drug not only provides hemostasis, but also improves the depth and duration of anesthesia along with a reduction of systemic toxic effects in local infiltration anesthesia2. The hemodynamic effects of intravenous epinephrine infusion are well known and include elevations in heart rate (HR) and blood pressure (BP). During local infiltration anesthesia, even small doses of epinephrine can produce cardiovascular effects. These hemodynamic changes were observed following a submucosal injection of lidocaine containing 54 µg epinephrine; higher doses of epinephrine produced a greater hemodynamic response3. Previous studies have reported that hemodynamic changes were caused by the submucosal infiltration of epinephrine during surgery performed under general anesthesia. In addition to these hemodynamic effects, epinephrine also influences metabolism, including the stimulation of lipolysis and an increase in lactate and glucose levels4.
Hyperlactatemia has a wide spectrum of manifestations. Patients can present with fatigue or gastrointestinal complaints, such as abdominal pain, nausea, vomiting, or diarrhea. Severe lactic acidosis can have a more severe presentation, such as hypotension, altered mental status, dyspnea, and cardiac arrhythmias5. However, the development of lactic acidosis in patients receiving epinephrine for local infiltration anesthesia or vasoconstriction is seldom recognized clinically. Only one case study concerning significant glycogenolysis, lactic acidosis, and hypokalemia as well as hemodynamic changes in patients undergoing a cervical cone biopsy under epidural anesthesia was found in the literature6.
There have been no reports concerning lactic acidosis induced by the submucosal injection or application of epinephrine as a vasoconstrictor in young, healthy patients undergoing orthognathic surgery under general anesthesia with serial follow-up of the perioperative lactate level. Therefore, we present such two cases.
A 25-year-old, 63-kg male patient was scheduled to undergo Le Fort I osteotomy, bilateral sagittal split osteotomy (BSSO), and genioplasty for the correction of mandibular prognathism with midface deficiency. A tonsillectomy had been performed in the same patient 10 years previously under general anesthesia without incident. His medical history was unremarkable. At the time of surgery, he was taking no medications and reported no allergies. The preanesthetic evaluation findings were all within normal limits.
The patient was not premedicated, and his preanesthetic vital signs were BP of 135/80 mmHg, HR of 87 beats per minute (bpm), and pulse oxygen saturation (SpO2) of 99%. General anesthesia was induced with intravenous thiopental sodium (300 mg), 2% lidocaine (60 mg), rocuronium (50 mg), and remifentanil (0.15 µg/kg/min), and a tracheal tube was inserted nasotracheally. General anesthesia was maintained with sevoflurane (1.5-3.0 vol%), O2 (1.5 L/min), N2O (1.5 L/min), and remifentanil (0.05-0.15 µg/kg/min). After performing a modified Allen's test to assess the collateral blood flow to the hand, a left radial artery catheter was inserted to maintain continuous arterial pressure monitoring and arterial blood gas sampling. Following induction, the patient' s arterial blood gas analysis (ABGA) results were pH 7.47, PaO2 324 mmHg, PaCO2 40 mmHg, HCO3– 29.1 mEq/L, base excess (BE) 5.4 mEq/L, glucose 102 mg/dL, lactate 1 mmol/L, and normal electrolytes.
A submucosal injection of 2% lidocaine with 1:100,000 epinephrine was administered into the upper vestibular area for local infiltration anesthesia before the first surgical incision was made. During the operation, additional injections were administered in both the retromolar area and in the lower anterior vestibule before each surgical incision. In total, 18 mL of 2% lidocaine with 1:100,000 epinephrine (180 µg epinephrine) were administered submucosally. Additionally, gauze soaked in 25 mL of 0.01% epinephrine was used for dense packing on the operative site, which exhibited frequent bleeding. The packing had to be left in place for 5 to 10 minutes until the bleeding stopped. During most of the procedure, the patient's vital signs were BP of 115-148 mmHg/45-65 mmHg and HR of 82-110 bpm. However, BP and HR increased to 155-185 mmHg/65-85 mmHg and 115-120 bpm, respectively, after intermittent injections of lidocaine containing 1:100,000 epinephrine. At this time, a calcium channel blocker, an ultra-short-acting β-blocker, and an increase in the remifentanil infusion were applied to correct his vital signs.
One hour twenty minutes after the first incision, the ABGA revealed the following: pH 7.29, PaCO2 43 mmHg, PaO2 288 mmHg, HCO3– 20.7 mEq/L, BE –5.9 mEq/L, glucose 274 mg/dL, and lactate 7.7 mmol/L. These results indicated metabolic acidosis, hyperglycemia, and hyperlactatemia, which became more severe over time. According to the serial ABGA measurements obtained during the operation, the minimal pH was 7.19, the HCO3– and BE decreased to 17.6 mEq/L and –10.6 mEq/L, respectively, and the glucose and lactate increased to 338 mg/dL and 11.6 mmol/L. The surgery concluded uneventfully. The patient was transferred to the intensive care unit (ICU) in a sedated and intubated state. Postoperatively, the serial ABGA findings improved, returning to normal 13 hours after the end of the surgery with fluid administration.(Table 1) He was extubated 21 hours after surgery end and discharged from the hospital without any problems.
A 25-year-old, 60-kg male patient was scheduled to undergo high Le Fort I osteotomy, BSSO, genioplasty, and malar augmentation for a mandibular prognathism with midface deficiency. His medical history was unremarkable except for a history of pneumonia two years previous. The patient was taking no medications and reported no allergies. The preanesthetic evaluation findings were all normal.
He was not premedicated, and his preanesthetic vital signs were BP of 128/70 mmHg, HR of 98 bpm, and SpO2 of 99%. General anesthesia was induced with intravenous thiopental sodium (300 mg), 2% lidocaine (60 mg), rocuronium (50 mg), and remifentanil (0.15 µg/kg/min), and nasotracheal intubation was performed. General anesthesia was maintained with sevoflurane (1.5-2.5 vol%), O2 (1.5 L/min), N2O (1.5 L/min), and remifentanil (0.05-0.13 µg/kg/min). After radial artery catheterization, the patient's first ABGA results were pH 7.44, PaO2 350 mmHg, PaCO2 37 mmHg, HCO3– 25.1 mEq/L, BE 0.9 mEq/L, glucose 95 mg/dL, lactate 1.6 mmol/L, and normal electrolytes.
Submucosal infiltration of 2% lidocaine with 1:100,000 epinephrine was performed in the upper vestibular area before the first surgical incision. During the operation, an additional injection was administered in the lower vestibule before each surgical incision. In all, 18 mL of 2% lidocaine along with 1:100,000 epinephrine (180 µg epinephrine) were injected submucosally. In addition, gauze soaked with 25 mL of 0.01% epinephrine was used for dense packing at the bleeding site in the operative field until the bleeding stopped. Intraoperatively, the patient's vital signs were BP of 105-140 mmHg/50-70 mmHg and HR of 95-110 bpm. However, the patient's BP and HR increased to 160-170 mmHg/65-100 mmHg and 105-128 bpm, respectively, after intermittent injections of lidocaine containing 1:100,000 epinephrine. To maintain stable vital signs, a calcium channel blocker and dose increases of remifentanil were applied.
One hour after the first incision, the ABGA was pH 7.3, PaCO2 43 mmHg, PaO2 296 mmHg, HCO3– 21.2 mEq/L, BE –5.2 mEq/L, glucose 277 mg/dL, and lactate 5.3 mmol/L. These findings implied the presence of lactic acidosis and hyperglycemia. After the first hour, ABGA measurements were repeated at one-hour intervals during the procedure. The ABGA results worsened over time, showing maximum change three hours after the surgery began. The pH, HCO3–, and BE decreased to pH 7.29, 18.3 mEq/L, and –8.3 mEq/L, respectively, while the glucose and lactate increased to 331 mg/dL and 9.9 mmol/L. The surgery was successful, and the patient was transferred to the ICU in a sedated and intubated state. Serial ABGA findings subsequently improved dramatically and returned to normal two hours after the end of the surgery with fluid resuscitation.(Table 2) He was extubated 19 hours after surgery end and discharged from the hospital with no complications.
In orthognathic surgery, local anesthetic solutions containing epinephrine are routinely administered submucosally in the operative field. Epinephrine is the main vasoconstrictor used in dental practice today123. In commonly used dental cartridges, the concentration of epinephrine varies from 1:50,000 (20 µg/mL) to 1:200,000 (5 µg/mL)2. Epinephrine delays the absorption of local anesthetics, which have vasodilating properties and rapid diffusing action. It also controls bleeding at incision sites and produces preemptive analgesia1237.
This vasoconstriction is due to the α-adrenergic receptor activation of epinephrine on peripheral blood vessels, most notably under the skin and within the mucous membranes. However, it also activates cardiac β1-adrenergic receptors, which increase the HR, BP, contractility, and myocardial oxygen consumption, and skeletal muscle β2-adrenergic receptors, leading to vasodilation of blood vessels23.
The metabolic effects of epinephrine include insulin resistance, hyperglycemia, hyperlactatemia, and the stimulation of lipolysis. Epinephrine directly induces lipolysis and increases the concentration of free fatty acids. Inhibition by epinephrine of glycogen synthesis in the skeletal muscle and liver likely accounts for this hyperglycemia and insulin resistance46. In addition, the development of hyperlactatemia is associated with hyperglycemia and pyruvate generation in the presence of pyruvate dehydrogenase inhibition. This mechanism is attributed to the epinephrine effect because β2-adrenergic receptors stimulate aerobic glycolysis through Na-K-ATPase activation in skeletal muscle. The associated enhanced glycolysis promotes pyruvate generation; pyruvate is later converted to lactate489.
The sedation induced by general anesthesia and remifentanil infusion has the advantage of suppressing the plasma epinephrine, norepinephrine, and hemodynamic responses to surgical stress. However, concomitant administration of a local anesthetic containing epinephrine negates these advantages. The sumucosal infiltration of epinephrine in orthognathic surgery significantly elevates the plasma epinephrine concentration due to the exogenous epinephrine rather than an adrenal release produced by the surgical stress10. In addition, submucosal injections of lidocaine, which contains epinephrine, are rapidly absorbed into the blood during general anesthesia, producing a higher plasma concentration than observed during conscious conditions7. Therefore, although elevating the plasma epinephrine is not identical to increasing sympathetic tone10, we assumed that the elevated plasma epinephrine level induced by the submucosal infiltration of epinephrine during surgery directly stimulated the hemodynamic and metabolic changes in these cases. In addition, the topical application of epinephrine-soaked gauze to the mucosa can induce the absorption of epinephrine into the systemic circulation and produce the observed hemodynamic effects11. Thus, it seems that a significant amount of epinephrine was absorbed via the submucosal injections and topical applications in these cases.
Lactic acidosis (lactate >5 mmol/L and pH <7.35) is classified as either type A or type B. Type A lactic acidosis is produced in response to a hypoxic or hypoperfusion state. In the absence of oxygen, pyruvate dehydrogenase is inhibited, preventing the conversion of pyruvate to acetyl-CoA and forcing pyruvate to undergo an anaerobic metabolism and be converted into lactate. In contrast, type B lactic acidosis is generated in the absence of hypoxia or hypoperfusion. Pyruvate is converted to lactate by way of aerobic glycolysis. The common causes of type B lactic acidosis are drugs, malignancies, and inborn errors of metabolism89.
The patients in these cases were young and healthy, and they had not received any other drug commonly recognized as a cause of lactic acidosis prior to the surgery. Their liver and kidney functions were normal, and there was no evidence of global hypoxia or hypoperfusion during the perioperative management. The patients had no factors that could have caused lactic acidosis except epinephrine; the lactic acidosis resolved spontaneously within hours after the cessation of epinephrine infiltration. Therefore, epinephrine-induced lactate released from the skeletal muscle and mediated by β2-adrenergic receptors may be a prime mechanism underlying lactic acidosis. It is known that the benign, self-limiting type B acidosis almost never causes a lactate level greater than 10 mmol/L9. Our patients developed elevated lactate levels that did not exceed 11.6 mmol/L and 9.9 mmol/L, respectively. However, regional hypoxia or hypoperfusion may not be detectable using systemic parameters alone. Future studies should investigate whether depressed mucosal blood flow or peripheral vasoconstriction induced by epinephrine in the operative field can produce lactic acidosis in patients undergoing orthognathic surgery under general anesthesia.
Higher concentrations of epinephrine may provide better hemostasis when infiltrated directly into the operative field3. Unfortunately, this practice also increases the likelihood of systemic effects of epinephrine in a dose-dependent manner10. There has been much debate about the maximum recommended dose of epinephrine as a local anesthetic. In 1984, the American Dental Association recommended 200 µg as the maximum dose for healthy patients12. High doses can produce detrimental effects, especially in patients with significant cardiovascular disease; therefore, the maximum recommended dose of epinephrine for cardiac patients is 40 µg231013. Additionally, if concerns exist regarding the systemic effects of epinephrine, the following methods can reduce the risk of adverse events: minimize the administration of epinephrine when possible, closely monitor the patient's vital signs, carefully administer the drug using preliminary aspiration to avoid inadvertent intravascular injection, and readminister additional doses with subsequent monitoring of vital signs if the patient's vital signs are stable2313. In general, most systemic effects of epinephrine are observed within 5 to 10 minutes of its injection, and the action duration of systemic epinephrine is approximately 10 to 15 minutes23. Therefore, careful observation for hemodynamic and metabolic changes in patients is essential during this period. In addition, the activity of drug-metabolizing enzymes tends to be reduced at low core temperatures. Accordingly, it is important to maintain a normothermic state when using a high dose of epinephrine.
The use of sodium bicarbonate to correct lactic acidosis remains controversial because sodium bicarbonate can increase lactate production14. Recent studies have suggested that therapy be guided by changes in lactate level15. Resuscitative efforts should be complemented to treat any underlying causes of lactic acidosis16. Our two patients were in healthy condition with unremarkable medical history, and we assumed the cause of lactic acidosis was excessive epinephrine use. Therefore, we only observed the patients with careful monitoring of their vital signs and fluid administration.
In conclusion, these cases suggest that the submucosal injection of a submaximal dose of a lidocaine containing epinephrine along with the topical application of epinephrinesoaked gauze applied directly to the mucosa can induce significant metabolic effects, such as lactic acidosis and hyperglycemia, as well as hemodynamic effects, including hypertension and tachycardia, which resolve rapidly without additional treatment following surgery in healthy patients undergoing orthognathic surgery with general anesthesia. Although these effects are unlikely to be serious in healthy patients, local anesthetics containing epinephrine should be used with caution, especially in patients with cardiovascular diseases, acid-base abnormalities, and disorders that could lead to hyperlactatemia or hyperglycemia. Furthermore, careful observation for changes in plasma lactate and glucose levels through serial follow-up of ABGA along with the intensive monitoring of vital signs should be performed in the perioperative period if epinephrine was used excessively as a vasoconstrictor during surgery. At the same time, deliberate hypotensive anesthesia (systolic BP remaining below 100 mmHg or mean arterial pressure [MAP] 30% below a patient's baseline MAP with a minimum MAP of 50-65 mmHg) could be an alternative to reduce the use of epinephrine as a vasoconstrictor and the loss of blood during surgery.
References
1. Sivanmalai S, Annamalai S, Kumar S, Prince CN, Chandrakala , Thangaswamy V. Pharmacodynamic responses of exogenous epinephrine during mandibular third molar surgery. J Pharm Bioallied Sci. 2012; 4(Suppl 2):S390–S393. PMID: 23066296.

2. Haas DA. An update on local anesthetics in dentistry. J Can Dent Assoc. 2002; 68:546–551. PMID: 12366885.
3. Becker DE, Reed KL. Local anesthetics: review of pharmacological considerations. Anesth Prog. 2012; 59:90–101. quiz 102-3. PMID: 22822998.

4. Gjedsted J, Buhl M, Nielsen S, Schmitz O, Vestergaard ET, Tønnesen E, et al. Effects of adrenaline on lactate, glucose, lipid and protein metabolism in the placebo controlled bilaterally perfused human leg. Acta Physiol (Oxf). 2011; 202:641–648. PMID: 21624100.

5. Schambelan M, Benson CA, Carr A, Currier JS, Dubé MP, Gerber JG, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr. 2002; 31:257–275. PMID: 12439201.

6. Cunningham AJ, Donnelly M, Bourke A, Murphy JF. Cardiovascular and metabolic effects of cervical epinephrine infiltration. Obstet Gynecol. 1985; 66:93–98. PMID: 4011076.

7. Homma Y, Ichinohe T, Kaneko Y. Oral mucosal blood flow, plasma epinephrine and haemodynamic responses after injection of lidocaine with epinephrine during midazolam sedation and isoflurane anaesthesia. Br J Anaesth. 1999; 82:570–574. PMID: 10472225.

8. Matejovic M, Radermacher P, Fontaine E. Lactate in shock: a highoctane fuel for the heart? Intensive Care Med. 2007; 33:406–408. PMID: 17242932.
9. Chi SJ, Stein E, Chaney MA, Ranucci M, Wall MH. Case 5--2009: severe lactic acidosis during cardiac surgery. J Cardiothorac Vasc Anesth. 2009; 23:711–719. PMID: 19789058.

10. Yagiela JA. Vasoconstrictor agents for local anesthesia. Anesth Prog. 1995; 42:116–120. PMID: 8934977.
11. Kameyama K, Watanabe S, Kano T, Kusukawa J. Effects of nasal application of an epinephrine and lidocaine mixture on the hemodynamics and nasal mucosa in oral and maxillofacial surgery. J Oral Maxillofac Surg. 2008; 66:2226–2232. PMID: 18940484.

12. Troullos ES, Goldstein DS, Hargreaves KM, Dionne RA. Plasma epinephrine levels and cardiovascular response to high administered doses of epinephrine contained in local anesthesia. Anesth Prog. 1987; 34:10–13. PMID: 3472472.
13. Kothari D, Abbas H. How safe is therapeutic dose of lignocaine with epinephrine: an overview. Natl J Maxillofac Surg. 2015; 6:132. PMID: 26668471.

14. Rachoin JS, Weisberg LS, McFadden CB. Treatment of lactic acidosis: appropriate confusion. J Hosp Med. 2010; 5:E1–E7.

15. Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, et al. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010; 182:752–761. PMID: 20463176.
16. Luft FC. Lactic acidosis update for critical care clinicians. J Am Soc Nephrol. 2001; 12(Suppl 17):S15–S19. PMID: 11251027.

Table 1
Serial arterial blood gas analysis during and after anesthesia in Case 1
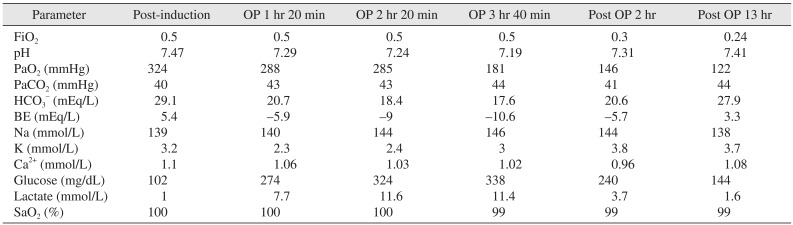
(BE: base excess, OP 1 hr 20 min: 1 hour 20 minutes after operation start, OP 2 hr 20 min: 2 hours 20 minutes after operation start, OP 3 hr 40 min: 3 hours 40 minutes after operation start, Post OP 2 hr: 2 hours after the end of the operation, Post OP 13 hr: 13 hours after the end of the operation)
Table 2
Serial arterial blood gas analysis during and after anesthesia in Case 2





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download