Abstract
Chondrosarcoma is a malignant tumor that originates from cartilaginous cells and is characterized by cartilage formation. Only 5% to 10% of chondrosarcoma occurs in the head and neck area, and it is uncommon in the temporomandibular joint area. This report describes an unusual case with a rare, large chondrosarcoma in a 47-year-old woman who presented with painless swelling and trismus. Computed tomography showed a large mass approximately 8.5×6.0 cm in size arising adjacent to the lateral pterygoid plate and condyle. There were features suggestive of bone resorption. The tumor was resected in a single block with perilesional tissues, and a great auricular nerve graft was performed because of facial nerve sacrifice. Microscopic examination of sections stained with H&E revealed chondrocytes with irregular nuclei and heterogeneous hyper chromatic tumor cells embedded in the chondrocyte lacuna. The diagnosis was a grade I chondrosarcoma. There was no evidence of recurrence at the 8-month follow-up, and a reconstruction surgery with fibular osteocutaneous free flap was performed. We report this unusual entity and a review of the literature.
Chondrosarcoma is a malignant tumor that originates from cartilaginous cells and is characterized by the formation of cartilage, but not bone1. It appears as a common primary bone tumor of the pelvis, ribs, and femur, but is rare in the head and neck regions2. Therefore, chondrosarcoma of the jaw is uncommon in the head and neck, but when present, it and primarily occurs at the anterior maxilla in the head and neck area or in the nasal cartilage. Chondrosarcoma in the temporomandibular joint (TMJ) area is exceptionally rare, and only 30 cases have been reported in the literature234567891011121314151617181920212223242526272829303132333435363738. Treatment of chondrosarcoma of the TMJ is often challenging and might be related to the presence of associated vital structures, which include the facial nerve, parotid gland, and the close proximity to the cranial base. Herein we present a case of a large chondrosarcoma in the TMJ area.
A 47-year-old woman with right facial swelling, suddenonset occlusal discrepancy, and trismus with a lobulated mass in the right preauricular area was referred to our clinic.(Fig. 1. A-C; The clinical and radiographic data have used under the agreement of her.) The patient had no spontaneous pain or facial paralysis. Clinically, the lesion was hard and tender and covered with normal skin. Computed tomography showed a large mass approximately 8.5×6.0 cm in size arising adjacent to the lateral pterygoid plate and condyle, with thinning of the cranial base and involving both the right lateral pterygoid and medial pterygoid muscles. Bone resorption from the right condyle and pterygoid plate was also observed.(Fig. 1. D-G) There was no significant lymph node enlargement detected either on clinical or radiologic analysis. Open biopsy was performed using a preauricular incision. On histology, cartilage cells were variable and bizarre. Heterogeneous tumor cells with cellular polymorphism were embedded in the chondrocyte lacuna in the mucinous or chondroid stroma. Based on the above findings, a diagnosis of grade 1 chondrosarcoma was made.
Surgical treatment was planned; due to the large mass involving the condyle, glenoid fossa, and cranial base with adjacent tissues, an extraoral approach with an extended preauricular incision, combined with a submandibular incision was used. The mass was dissected and surgically resected from adjacent perilesional tissue in a single block, and portions of the facial nerve involved in the mass were sacrificed. During surgery, for functional reconstruction of the facial nerve, the great auricular nerve was grafted to the damaged facial nerve. The auricular nerve graft was intentionally cut and ligated to both ends of the damaged facial nerve under microscopy. Part of the cranial bone base was also resected; fortunately, there was no invasion of the dura.(Fig. 2) The excised mass measured 8.5×6.5 cm in size with an intact synovial membrane and disk, and there appeared to be glistening cartilaginous tissue with a grayish-white pattern on the cut surface.(Fig. 3. A)
After surgery, the patient experienced anesthesia in the temporal, buccal, and zygomatic branch regions and hypoesthesia in the mandibular and cervical branch. The motor nerve was also damaged; thus, movement of the right side of the forehead and corner of the mouth was barely detectable, but she could close her right eye, although this occurred much slower compared to the left eye.
Microscopic examination of sections stained with H&E revealed chondrocytes with irregular nuclei. Heterogeneous tumor cells embedded in the chondrocyte lacuna in the mucinous or chondroid stroma were also observed. The cells were composed of a diffuse proliferation of atypical chondrocytic cells in a myxoid or chondroid matrix where granular calcified materials were scattered and showed cellular pleomorphism with a bizarre nucleus appearance. Mitosis was observed in some areas.(Fig. 3. B, 3. C) Histological diagnosis was a low grade (grade I) chondrosarcoma without perineural, vascular, or lymph node invasion.
Due to the large size of the tumor, it was difficult to obtain sufficient marginal clearance. The patient was referred to a radiation oncologist for adjunctive radiation therapy due to the close margin. The radiation was delivered in a daily single dose of 1.8 Gy, total dose of 50.4 Gy on the right parapharyngeal space and a total dose of 10.8 Gy on the right infratemporal fossa area. After radiation therapy, radiographic and clinical examinations during the follow-up period showed no signs of recurrence. The patient is a young woman, and esthetic outcomes were very important for her. She desired reconstructive surgery to address the soft tissue defect in the right preauricular area as early as possible.(Fig. 4) Subsequently, reconstruction was performed with a fibular osteocutaneous free flap 8 months after the first surgery. The condyle was reconstructed with fibular bone, and the soft tissue depression in the preauricular area was augmented with a vascularized flap. Facial nerve weakness improved clinically as the damaged nerve and grafted preauricular nerve regenerated and healed.(Fig. 5) An electromyogram was needed to evaluate the facial nerve but was not performed because the patient refused any examination of the facial nerve.
Chondrosarcoma is a malignant tumor that originates in cartilaginous cells and that remains malignant throughout its evolution. Chondrosarcoma represents 10% to 20% of all malignant bone tumors, and only 1% to 12% originate in the head and neck region34. The most common site in the head and neck is the larynx, followed by the mandible, nasal cavity, and maxilla5. Chondrosarcoma in the TMJ area is rare, with only 30 cases reported in the literature234567891011121314151617181920212223242526272829303132333435363738; all are presented in Table 1.
In previous reports, the mean age of patients with chondrosarcoma in the TMJ area was 38 to 48 years with no or slight female sex predominance. These characteristics vary from chondrosarcomas that occur in the head and neck area1533.
A major symptom of chondrosarcoma in the TMJ area is swelling in the preauricular region, followed by pain and trismus5. The duration of symptoms before final diagnosis is generally 3 to 24 months17, although several studies have reported a duration of 6 to 8 years23.
For accurate diagnosis, conventional radiographic investigation and computed tomography are helpful, but there are no unique pathognomonic findings in chondrosarcoma34, which appears as a mass with single or multiple radiolucent areas that can involve a calcified mass with a condylar deformity, bone destruction, erosion of adjacent bone, condylar resorption, and sometimes cranial invasion. In most cases, increases in the articular space and the length of the condylar neck with radiopacity of the condyle are observed5. In many chondrosarcomas of the TMJ, radiographic evidence of widening of the joint space has been reported35. In this case report, joint space widening was also observed in computed tomography.
Histopathologically, chondrosarcoma of the TMJ area is similar to that of the head and neck areas or pelvis, ribs, or other regions. The lesions are microscopically lobulated with cellular neoplasms, hyaline cartilaginous proliferation, and sarcomatous stroma that contain stellate. The presence of mitotic figures is rare; therefore, their absence cannot rule out the diagnosis of chondrosarcoma. Criteria for diagnosis include an increase in the number of cells, expansion of cells, and formation of giant or binucleated cells3. Chondrosarcoma is classified into grade I, II, or III based on the frequency of mitosis, cellularity, and nucleus size36. However, distinguishing the grade is difficult because the grading system is derived from other joint chondrosarcomas, not solely from temporomandibular area chondrosarcomas20.
The reported 5-year survival rate is 44% to 54.6%, and 10-year rate is 28%32. Murayama et al.15 reported that 8 of 20 patients died 5 months to 6 years after primary treatment. Although Evans et al.36 reported that the pathological grade was a useful prognostic factor, Saito et al.33 reported that grade was not related to prognosis. Conversely, metastasis is related to histological grade; in grade II, the reported metastasis rate is 10%, while that in grade III is 71%. There were no reports of metastasis in grade I chondrosarcoma. Local recurrence is more common than distant metastasis and is related to tumor grade. Metastasis usually occurs in limbs and lungs, and regional lymph node metastasis is uncommon20. However, the most important factor in prognosis is resectability.
Wide local resection is the first choice of treatment, and neck node dissection is usually unnecessary because of the low incidence of regional lymph node metastasis37. Chondrosarcoma is traditionally regarded as radioresistant, and radiation therapy is best reserved for high-grade lesions. However, a recent study reported that it can be radiosensitive and potentially radiocurable. However, radiation therapy is generally used as an adjunct treatment and not as a single treatment modality. Thus, the primary role of irradiation is to treat unresectable areas or incompletely resected lesions after surgery. Use of chemotherapy is limited in chondrosarcoma but can be applied as an adjuvant therapy in cases with aggressive behavior, rapid local recurrence, or high grade38.
In other reports, the tumor size was usually less than 3.0 to 4.0 cm. One case reported a 5.0-cm sized mass that was reconstructed with a rectus free flap35. In our case, the lesion size was 8.5 cm, which is exceptionally large for oral and maxillofacial surgery, making it difficult to obtain appropriate marginal clearance. Resection resulted in a large defect on the face and associated esthetic and functional problems. Therefore, radiation therapy and reconstructive surgery were required.
In our case, an enlarging mass and widening of the joint space in computed tomography were helpful to diagnose the chondrosarcoma before open biopsy. Wide local resection was attempted, with facial nerve sacrifice, but it was difficult to obtain a clear margin due to the large-sized mass; adjunctive radiation therapy was performed, and there was no sign of recurrence after follow-up for 8 months. Because of the large mandible defect of the condyle, glenoid fossa, and soft tissue in the right preauricular area, we used a fibular osteocutaneous free flap to reconstruct the mandible. This flap has relatively low donor morbidity and provided a good esthetic outcome.
In conclusion, chondrosarcoma of the TMJ is a very rare event. In our patient, because of the large mass, which was related to glenoid fossa involvement, the cranial base, facial nerve, and condyle were also involved. Using an extraoral approach, the mass was dissected and resected in a single block, and the sacrificed facial nerve was repaired with a nerve graft. Reconstructive surgery was performed following radiation therapy, 8 months after the first surgery, using a fibular osteocutaneous free flap for optimal functional and esthetic results. The long-term prognosis and recovery of the facial nerve function will be reported in future work.
References
1. Schajowicz F. Histological typing of bone tumours. Berlin: New York: Springer-Verlag;1993. p. 128.
2. Burkey BB, Hoffman HT, Baker SR, Thornton AF, McClatchey KD. Chondrosarcoma of the head and neck. Laryngoscope. 1990; 100:1301–1305. PMID: 2243522.

3. Lee SY, Lim YC, Song MH, Seok JY, Lee WS, Choi EC. Chondrosarcoma of the head and neck. Yonsei Med J. 2005; 46:228–232. PMID: 15861495.

4. Weiss WW Jr, Bennett JA. Chondrosarcoma: a rare tumor of the jaws. J Oral Maxillofac Surg. 1986; 44:73–79. PMID: 3455727.

5. Gallego L, Junquera L, Fresno MF, de Vicente JC. Chondrosarcoma of the temporomandibular joint. A case report and review of the literature. Med Oral Patol Oral Cir Bucal. 2009; 14:E39–E43. PMID: 19114955.
6. Gingrass RP. Chondrosarcoma of the mandibular joint; report of case. J Oral Surg (Chic). 1954; 12:61–63. PMID: 13118419.
7. Lanier VC Jr, Rosenfeld L, Wilkinson HA 3rd. Chondrosarcoma of the mandible. South Med J. 1971; 64:711–714. PMID: 5089146.

8. Sudo Y, Kusano A, Nagabatake S, Komai M, Idezaki K, Nishijima K. Two cases of chondrosarcoma of the mandible. JJ SS. 1972; 211:417. (Abstract).
9. Richter KJ, Freeman NS, Quick CA. Chondrosarcoma of the temporomandibular joint: report of case. J Oral Surg. 1974; 32:777–781. PMID: 4528470.
10. Tullio G, D'Errico P. Chondrosarcoma of the mandible. Clinical andhistological considerations. Ann Stomatol (Roma). 1974; 23:191–206. PMID: 4535384.
11. Kato Y, Ogino M, Furutani M, Asai H, Hachisuga Y, Igarashi M. Malignant tumor of the mandible: chondrosarcoma. Orthopedic Surg Tokyo. 1975; 26:313.
12. Nortjé CJ, Farman AG, Grotepass FW, Van Zyl JA. Chondrosarcoma of the mandibular condyle. Report of a case with special reference to radiographic features. Br J Oral Surg. 1976; 14:101–111. PMID: 1070334.

13. Cadenat H, Combelles R, Fabert G, Clouet M. Chondrosarcoma of the condyle (author's transl). Rev Stomatol Chir Maxillofac. 1979; 80:20–22. PMID: 289160.
14. Morris MR, Clark SK, Porter BA, Delbecq RJ. Chondrosarcoma of the temporomandibular joint: case report. Head Neck Surg. 1987; 10:113–117. PMID: 3507419.

15. Murayama S, Suzuki I, Nagase M, Shingaki S, Kawasaki T, Nakajima T, et al. Chondrosarcoma of the mandible. Report of case and a survey of 23 cases in the Japanese literature. J Craniomaxillofac Surg. 1988; 16:287–292. PMID: 3049678.
16. Wasenko JJ, Rosenbloom SA. Temporomandibular joint chondrosarcoma: CT demonstration. J Comput Assist Tomogr. 1990; 14:1002–1003. PMID: 2229544.
17. Nitzan DW, Marmary Y, Hasson O, Elidan J. Chondrosarcoma arising in the temporomandibular joint: a case report and literature review. J Oral Maxillofac Surg. 1993; 51:312–315. PMID: 8445475.

18. Merrill RG, Yih WY, Shamloo J. Synovial chondrosarcoma of the temporomandibular joint: a case report. J Oral Maxillofac Surg. 1997; 55:1312–1316. PMID: 9371126.

19. Giraud O, Lockhart R, Dichamp J, Capelle L, Kujas M, Dupuis HJ, et al. Chondrosarcoma of the temporomandibular joint. Apropos of a case and review of the literature. Rev Stomatol Chir Maxillofac. 1997; 98:2–6. PMID: 9273672.
20. Sesenna E, Tullio A, Ferrari S. Chondrosarcoma of the temporomandibular joint: a case report and review of the literature. J Oral Maxillofac Surg. 1997; 55:1348–1352. PMID: 9371134.

21. Ichikawa T, Miyauchi M, Nikai H, Yoshiga K. Synovial chondrosarcoma arising in the temporomandibular joint. J Oral Maxillofac Surg. 1998; 56:890–894. PMID: 9663582.

22. Batra PS, Estrem SA, Zitsch RP, McDonald R, Ditto J. Chondrosarcoma of the temporomandibular joint. Otolaryngol Head Neck Surg. 1999; 120:951–954. PMID: 10352461.

23. Mostafapour SP, Futran ND. Tumors and tumorous masses presenting as temporomandibular joint syndrome. Otolaryngol Head Neck Surg. 2000; 123:459–464. PMID: 11020186.

24. Angiero F, Vinci R, Sidoni A, Stefani M. Mesenchymal chondrosarcoma of the left coronoid process: report of a unique case with clinical, histopathologic, and immunohistochemical findings, and a review of the literature. Quintessence Int. 2007; 38:349–355. PMID: 17432791.
25. Yun KI, Park MK, Kim CH, Park JU. Chondrosarcoma in the mandibular condyle: case report. J Korean Assoc Oral Maxillofac Surg. 2008; 34:95–98.
26. Oliveira RC, Marques KD, Mendonça AR, Mendonça EF, Silva MR, Batista AC, et al. Chondrosarcoma of the temporomandibular joint: a case report in a child. J Orofac Pain. 2009; 23:275–281. PMID: 19639107.
27. Garzino-Demo P, Tanteri G, Boffano P, Ramieri G, Pacchioni D, Maletta F, et al. Chondrosarcoma of the temporomandibular joint: a case report and review of the literature. J Oral Maxillofac Surg. 2010; 68:2005–2011. PMID: 20537779.

28. González-Pérez LM, Sánchez-Gallego F, Pérez-Ceballos JL, López-Vaquero D. Temporomandibular joint chondrosarcoma: case report. J Craniomaxillofac Surg. 2011; 39:79–83. PMID: 20456965.

29. Ramos-Murguialday M, Lasa-Menéndez V, Ignacio Iriarte-Ortabe J, Couce M. Chondrosarcoma of the mandible involving angle, ramus, and condyle. J Craniofac Surg. 2012; 23:1216–1219. PMID: 22801134.

30. Abu-Serriah M, Ahluwalia K, Shah KA, Bojanic S, Saeed N. A novel approach to chondrosarcoma of the glenoid fossa of the temporomandibular joint: a case report. J Oral Maxillofac Surg. 2013; 71:208–213. PMID: 22749519.

31. Giorgione C, Passali FM, Varakliotis T, Sibilia M, Ottaviani F. Temporo-mandibular joint chondrosarcoma: case report and review of the literature. Acta Otorhinolaryngol Ital. 2015; 35:208–211. PMID: 26246667.
32. MacIntosh RB, Khan F, Waligora BM. Chondrosarcoma of the temporomandibular disc: behavior over a 28-year observation period. J Oral Maxillofac Surg. 2015; 73:465–474. PMID: 25577455.
33. Saito K, Unni KK, Wollan PC, Lund BA. Chondrosarcoma of the jaw and facial bones. Cancer. 1995; 76:1550–1558. PMID: 8635057.

34. Saini R, Abd Razak NH, Ab Rahman S, Samsudin AR. Chondrosarcoma of the mandible: a case report. J Can Dent Assoc. 2007; 73:175–178. PMID: 17355810.
35. Nomura T, Kobayashi T, Shingaki S, Saito C. A case of chondrosarcoma arising in the temporomandibular joint. Case Rep Otolaryngol. 2015; 2015:832532. PMID: 25688316.

36. Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977; 40:818–831. PMID: 890662.
37. Koch BB, Karnell LH, Hoffman HT, Apostolakis LW, Robinson RA, Zhen W, et al. National cancer database report on chondrosarcoma of the head and neck. Head Neck. 2000; 22:408–425. PMID: 10862026.

38. Harwood AR, Cummings BJ, Fitzpatrick PJ. Radiotherapy for unusual tumors of the head and neck. J Otolaryngol. 1984; 13:391–394. PMID: 6100552.
Fig. 1
A-C. Frontal view of the patient before tumor resection. Right-side preauricular swelling, occlusal discrepancy and trismus on presentation. D-G. Low-enhanced tumor around the right condylar head on contrast-enhanced computed tomography and magnetic resonance imaging.
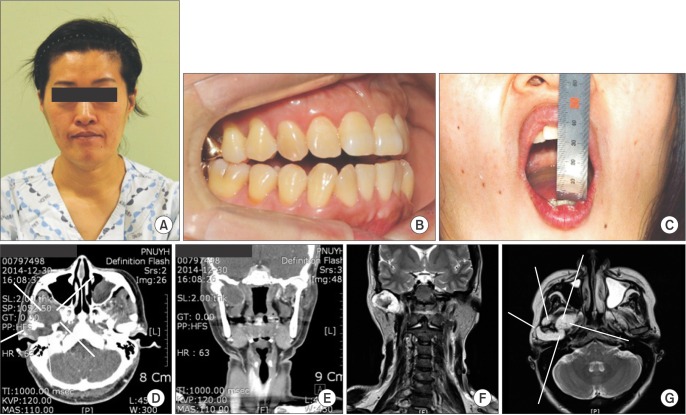
Fig. 2
The surgical process. An extraoral approach with an extended preauricular incision and submandibular incision was used to dissect and resect the large mass. The glenoid fossa and cranial base with adjacent tissues, facial nerve, and condyle were involved in the mass.
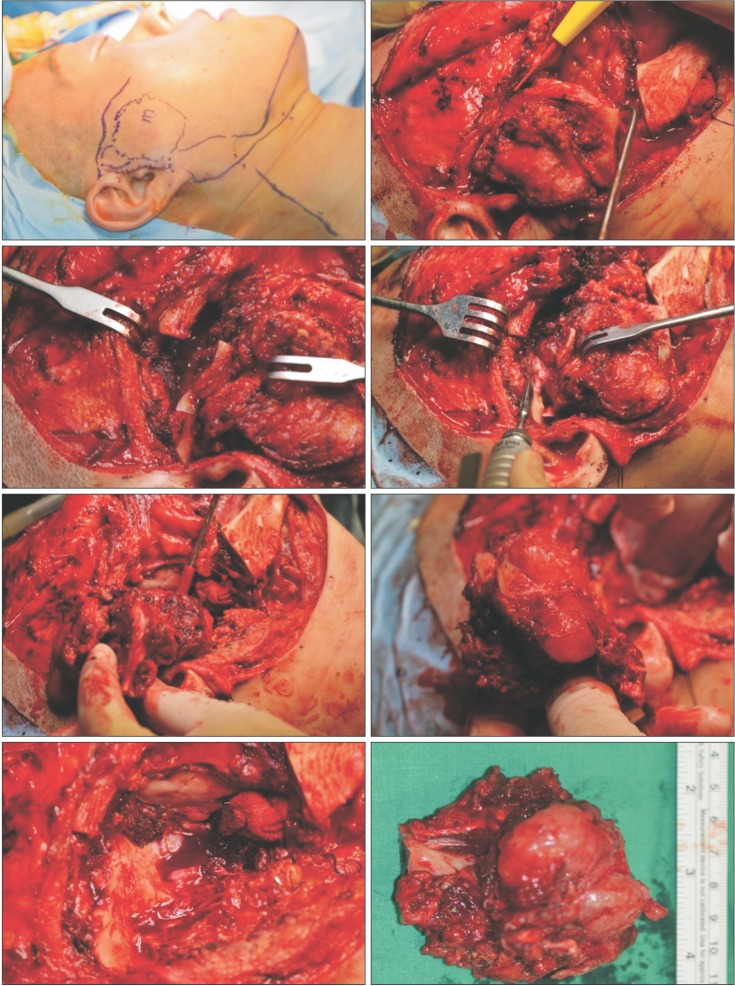
Fig. 3
A. Resected mass with the right condylar head. B, C. Atypical chondrocytic cells in myxoid or chondroid matrix (B; H&E staining, ×400), pleomorphism with bizarre nucleus appearance (C; H&E staining, ×50).
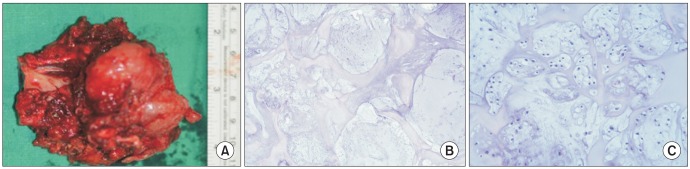
Fig. 4
Frontal view of the patient after tumor resection and before reconstruction. There was a soft tissue depression on the right side of the preauricular area.

Fig. 5
Frontal view of the patient after reconstruction. The soft tissue in the preauricular area was augmented with a vascularized flap. The left two pictures show partial improvement in facial nerve weakness after great auricular nerve graft.
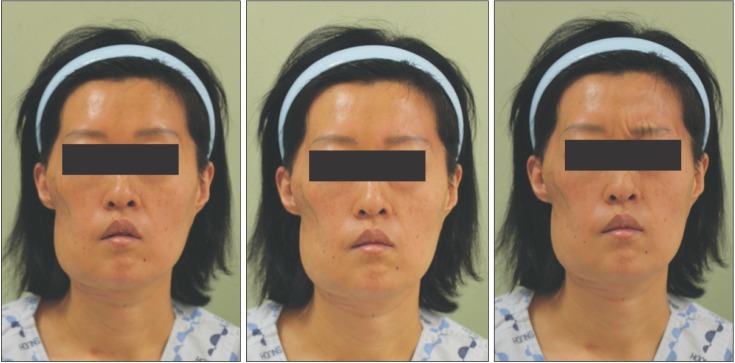
Table 1
Reported temporomandibular joint chondrosarcoma cases
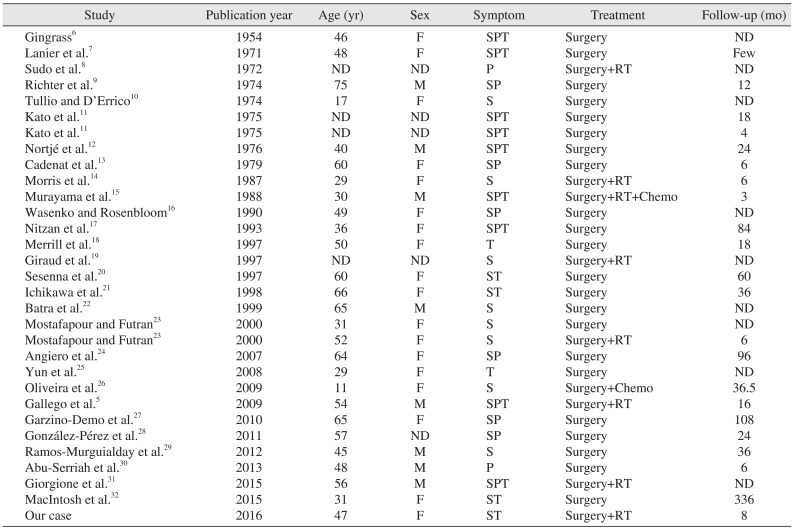
| Study | Publication year | Age (yr) | Sex | Symptom | Treatment | Follow-up (mo) |
|---|---|---|---|---|---|---|
| Gingrass6 | 1954 | 46 | F | SPT | Surgery | ND |
| Lanier et al.7 | 1971 | 48 | F | SPT | Surgery | Few |
| Sudo et al.8 | 1972 | ND | ND | P | Surgery+RT | ND |
| Richter et al.9 | 1974 | 75 | M | SP | Surgery | 12 |
| Tullio and D’Errico10 | 1974 | 17 | F | S | Surgery | ND |
| Kato et al.11 | 1975 | ND | ND | SPT | Surgery | 18 |
| Kato et al.11 | 1975 | ND | ND | SPT | Surgery | 4 |
| Nortjé et al.12 | 1976 | 40 | M | SPT | Surgery | 24 |
| Cadenat et al.13 | 1979 | 60 | F | SP | Surgery | 6 |
| Morris et al.14 | 1987 | 29 | F | S | Surgery+RT | 6 |
| Murayama et al.15 | 1988 | 30 | M | SPT | Surgery+RT+Chemo | 3 |
| Wasenko and Rosenbloom16 | 1990 | 49 | F | SP | Surgery | ND |
| Nitzan et al.17 | 1993 | 36 | F | SPT | Surgery | 84 |
| Merrill et al.18 | 1997 | 50 | F | T | Surgery | 18 |
| Giraud et al.19 | 1997 | ND | ND | S | Surgery+RT | ND |
| Sesenna et al.20 | 1997 | 60 | F | ST | Surgery | 60 |
| Ichikawa et al.21 | 1998 | 66 | F | ST | Surgery | 36 |
| Batra et al.22 | 1999 | 65 | M | S | Surgery | ND |
| Mostafapour and Futran23 | 2000 | 31 | F | S | Surgery | ND |
| Mostafapour and Futran23 | 2000 | 52 | F | S | Surgery+RT | 6 |
| Angiero et al.24 | 2007 | 64 | F | SP | Surgery | 96 |
| Yun et al.25 | 2008 | 29 | F | T | Surgery | ND |
| Oliveira et al.26 | 2009 | 11 | F | S | Surgery+Chemo | 36.5 |
| Gallego et al.5 | 2009 | 54 | M | SPT | Surgery+RT | 16 |
| Garzino-Demo et al.27 | 2010 | 65 | F | SP | Surgery | 108 |
| González-Pérez et al.28 | 2011 | 57 | ND | SP | Surgery | 24 |
| Ramos-Murguialday et al.29 | 2012 | 45 | M | S | Surgery | 36 |
| Abu-Serriah et al.30 | 2013 | 48 | M | P | Surgery | 6 |
| Giorgione et al.31 | 2015 | 56 | M | SPT | Surgery+RT | ND |
| MacIntosh et al.32 | 2015 | 31 | F | ST | Surgery | 336 |
| Our case | 2016 | 47 | F | ST | Surgery+RT | 8 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download