Abstract
Objectives
The purpose of this study was to investigate the effects of low-level laser therapy (LLLT) with a diode gallium-aluminum-arsenide (Ga-Al-As) low-level laser device on the healing and attachment of titanium implants in bone.
Materials and Methods
Thirteen New Zealand white male rabbits weighing 3.0±0.5 kg were used for this study. Dental titanium implants (3.75 mm in diameter and 8.5 mm in length, US II RBM plus fixture; Osstem, Seoul, Korea) were implanted into both femurs of each rabbit. The rabbits were randomly divided into a LLLT group and a control group. The LLLT was initiated immediately after surgery and then repeated daily for 7 consecutive days in the LLLT group. Six weeks and 12 weeks after implantation, we evaluated and compared the osseointegration of the LLLT group and control group, using histomorphometric analysis, removal torque testing, and resonance frequency analysis (RFA). The results were statistically significant when the level of probability was 0.05 or less based on a non-parametric Mann-Whitney U-test.
Results
The implant survival rate was about 96%. Histologically and histomorphometrically, we observed that the titanium implants were more strongly attached in LLLT group than in control group. However, there was no significant difference between the LLLT group and control group in removal torque or RFA.
Dental implants have been widely used as a solution for tooth loss. The success of a dental implant depends on osseointegration, direct adhesion of the implant surface to the bone. Good bone remodeling, favorable bone quality for dental implant placement, appropriate implant design and surface treatment, and appropriate surgical treatment are essential for achieving successful osseointegration. Application of low-level laser therapy (LLLT) for better osseointegration has been conducted in the field of implantology which has been developed by design of implant, surface treatment, and surgical methodology.
LLLT has been found to be effective for relieving pain, healing soft tissues, and in nerve damage recovery123. LLLT stimulates vascularization of the fracture site and bone defect and also stimulates osteoblasts, which can facilitate recovery of hard tissue. In addition, it has been recently reported that LLLT has positive effects in ossification and osseointegration of dental implants456. Research on the effects of LLLT on osseointegration, both in vitro and in vivo, has been conducted, although the results remain controversial.
In this research, we performed an animal study to determine if in vivo application of LLLT effectively stimulates osseointegration of dental implants. We performed histomorphometric analysis, removal torque testing, and resonance frequency analysis (RFA) to evaluate dental implant osseointegration and to ensure clinical results. Removal torque testing is a method that tests the strength of osseointegration and has been verified primarily through animal studies. RFA can measure early implant stability and regularly determine the status of an implant. The peak removal torque value measured by a digital torque machine is used for removal torque testing, and the implant stability quotient (ISQ) was statistically analyzed in this study for RFA.
We used various methodologies to analyze the effects of LLLT on the osseointegration of titanium implants in this animal study.
Thirteen white New Zealand rabbits (weight range, 2.5-3.5 kg) were tested and were fed pellets and water and house at a room temperature of 20℃. Dental implants were placed into each femur of the rabbits, and the rabbits were randomly divided into two groups. LLLT group was treated with LLLT for one week after the surgery, and the other was designated as the control group, which was not treated with LLLT. Twenty-six dental titanium implants (US II RBM plus fixture; Osstem, Seoul, Korea) were used, and a diode galliumaluminum-arsenide (Ga-Al-As) laser device (IMPRA-ORT; NDLUX, Anyang, Korea) was used for LLLT.
All experiments were approved by the Pusan National University Institutional Animal Care and Use Committee and complied with its standards.
Before surgery, ketamine chloride (35 mg/kg, Ketalar; Yuhan Co., Seoul, Korea) and xylazine hydrochloride (10 mg/kg, Rumpun; Bayer Korea, Seoul, Korea) were injected into the rabbit femurs for general anesthesia. After covering the skin with betadine, infiltration anesthesia was administered to the rabbit with 2% lidocaine (1:100,000 epinephrine; Yuhan Co.). A 1-cm long skin incision was made toward each lateral femoral condyle, and the periosteum was elevated so that the bone was exposed. An implant frame was built into the central area of the condyle using round burs that were 2.0 mm, 3.0 mm, and 3.5 mm in diameter at 1,500 rpm, and then a 3.75-mm diameter and 8.5-mm long implant was carefully placed. The periosteum and the muscles were then stitched with 4-0 polyglycolic acid, and the skin was stitched with 3-0 silk. To prevent infection after surgery, all of the rabbits were given intramuscular antibiotic injections (gentamycin 5 mg/kg; AJU Pharm Co., Seoul, Korea).
After implantation, the LLLT group was treated with LLLT, using a Ga-Al-As diode with a wavelength of 808 nm. The laser was applied to the tissue area surrounding the implants with 100 mW output and an 830 mW/cm2 power density for one minute. The LLLT was applied to the LLLT group for one week, while the control group did not receive any additional treatment. Six rabbits were sacrificed in the sixth week, and the remaining seven rabbits were sacrificed in the 12th week. The rabbits were clinically examined to determine whether the implants were successfully, and any unsuccessful implants were excluded from the analysis.
The ISQ value was measured using an RFA analyzer (Osstell mentor; Integration Diagnostics AB, Göteborg, Sweden). The initial ISQ value was measured immediately after the implants were placed, and the final ISQ value was measured before tissue extraction after sacrifice. For comparison, the difference between the values (final ISQ-initial ISQ) was converted into a percentage, written as dif. % ISQ.
After sacrifice, rabbits from each group were selected, and their peak removal torque values were measured when the implants were removed. An axial digital torque machine (MGT 12R; Mark-10 Co., Copiague, NY, USA) was used for measurement, and the automatically recorded values in the machine were used.
After the sixth and 12th weeks of implant placement, rabbits from each group were sacrificed, and their femoral condyle was amputated for extraction. The extracted tissues were fixed in 10% neutral buffered formalin for six weeks. The fixed tissues were decalcified using a decalcification solution of 10% ethylenediaminetetraacetic acid and NaOH for 12 weeks. Then, the tissues were placed in tissue capsules and washed in flowing water for 12 hours. After the dehydration and clearing with alcohol and paraffin, the tissues were paraffin embedded, and the team generated 4-µm thick serial sagittal sections. H&E and Masson's trichrome stains were performed on the sections, and the tissue samples were observed for histological findings with a light microscope. An image program (Zen 2011; Carl Zeiss Microscopy GmbH, Königsallee, Germany) was used to produce digital images, and the images were assessed for histomorphometric analysis.
The bone-to-implant contact (BIC) ratio was measured for histomorphometric analysis. The ratio of the total length of a screw thread to the length of the screw thread that contacted bone was converted into a percentage, which was referred to as the BIC ratio. Osseous tissues to the third screw thread of each implant were observed with the light microscope (×100, Carl Zeiss Microscopy GmbH). The Zen 2011 program (Carl Zeiss Microscopy GmbH) was used to calculate the values and the ratios.
After H&E staining, a light microscope program (Carl Zeiss Microscopy GmbH) was used to observe osteogenic cells and the extracellular matrix.
Masson's trichrome staining was used to observe collagen formation, osteogenic cells, and the extracellular matrix. To observe mineralized and unmineralized osteogenic cells, which are dependent on degree of formation of collagen, paraffin was removed from the 4-µm thick paraffin tissue sections in xylene, and the tissue sections were placed in bovine solution overnight. Then, the tissue sections were washed in flowing water until the yellow color disappeared. After washing several times in distilled water, the samples were subjected to nuclear staining in weight iron hematoxylin for 10 minutes. After the nuclear staining, the tissue sections were washed in flowing water for 10 minutes and stained with phosphomlybdic-phosphotungstic acid solution for 10 minutes. Then, the tissue sections were stained in aniline blue solution for another five minutes and washed with distilled water. After the processes, 1% acetic acid solution was applied to the tissues for 1 to 3 minutes, and the tissues were washed several times with distilled water. The tissues were then sealed after dehydration and clearing processes.
To determine the difference in osseointegration between the LLLT group and control group, BIC ratio values at the sixth and 12th weeks, removal torque values, and dif. % ISQ values were evaluated through non-parametric Mann Whitney U-testing (PASW Statistics for Windows version 18.0; IBM Co., Armonk, NY, USA) to determine the statistical significance. A P-value less than or equal to 0.05 was determined to be statistically significant.
The rabbits were healthy without any abnormalities, such as weight loss, and the surgical sites were healed without infection or wound. There was one failed implant, and the overall success rate of the 26 implants was 96%.
Tissue comparison in the six-week groups indicated that the LLLT group had more bone matrix around the implants than the control group.(Fig. 1. A, 1. B) Analysis also indicated that the LLLT group at 12 weeks had more bone matrix around the implants.(Fig. 1. C, 1. D)
Tissue comparison in the six-week groups indicated that the LLLT group had more collagen and unmineralized bone matrix than the control group.(Fig. 2. A, 2. B) The 12-week LLLT group also had significantly more collagen and unmineralized bone matrix than the control group. The difference between the six-week groups and the 12-week groups was fairly large.(Fig. 2. C, 2. D)
In histomorphometric analysis, the average BIC ratio values of the control group and the LLLT group at the sixth week were 52.1%±18.3% and 55.4%±9.0%, respectively, while those at the 12th week were 50.4%±9.8% and 62.2%±10.2%. There was no statistically significant difference (P>0.05) in average BIC ratio values of the time groups. However, the LLLT groups showed higher average values than the control groups.(Fig. 3)
For the six-week groups, removal torque values of the control group and the LLLT group were 60.0±32.5 cNm and 54.1±4.3 cNm, respectively. The LLLT group showed a lower average value than the control group, but there was no statistically significant difference (P>0.05). For the 12-week groups, the values were 37.4±6.1 cNm and 90.0±21.1 cNm, respectively, and the value of the LLLT group was significantly higher than that of the control group (P≤0.05).(Table 1)
The ISQ values in the six-week LLLT and control groups were 68.3±15.5 and 69.0±10.0, respectively, a difference that was not statistically significant (P>0.05). At the 12th week, the values were 73.3±6.6 and 65.7±9.7, respectively, and the difference between the LLLT group and the control group values was statistically significant (P≤0.05).(Table 2)
Laser therapy has been increasingly used in dentistry since 1964. A low-level laser (LLL) is defined as a laser that does not increase the temperature of tissue higher than 36.5℃, or normal body temperature, and LLL has been widely used in dental therapy for the last 30 years. LLLT is effective in relieving pain, healing wounds, and reducing inflammation. The main effect of LLLT is non-exothermic in vivo stimulation, and no side effects have been reported. The LLL wave-length is between 500 to 1,200 nm, and a Ga-As-Al diode laser, which was used in this study, has a wavelength of 808 nm. The Ga-As-Al diode laser has been found to have a better penetration effect than any other lasers.
Research on the effects of LLLT on bone fracture and defect has reported that LLLT has positive effects on dental implant osseointegration. According to Khadra7, a group of rabbits with implants placed in the tibia and that were treated with LLLT showed higher osseointegration than the control group of rabbits. At eight weeks after implantation, the LLLT group had higher measured values than the control group in the tensile pullout test and in histomorphometric analysis. According to Guzzardella et al.8, after three and six weeks of implantation in the fibulas, rabbits that were treated with LLLT had higher measured osseointegration than those that were not treated with LLLT. Additionally, Maluf et al.9 placed implants into the shinbones of white mice and applied an LLL of 48 J/cm2 to a group of mice. In that research, the LLL group had higher removal torque values than the control group. Kim et al.10 placed implants in white mice, applied LLL to a group of mice, performed immunohistochemistry tests such as receptor activator of nuclear factor-kappa B ligand, receptor activator of nuclear factor-kappa B, osteoprotegerin, and reported that the LLL group had better bone metabolism ability and showed improving osteoclastic activity.
However, in contrast, some reports have shown that LLLT has no beneficial effect or even has negative effects on osseointegration. Pereira et al.11 placed implants in the fibula of rabbits, and the analysis showed that the LLLT group and control group did not have statistically significant differences.
Similarly, results of research that examine the effects of LLLT on osseointegration vary, and the application of LLLT to clinical practices is still debated. This research study analyzed the effects of LLLT on osseointegration through histopathological analysis and by assessing the degree of osseointegration in an animal model.
Histopathological analysis of H&E tissue staining indicated that both the six- and 12-week LLLT groups had more bone matrix around the implants than the control groups. In Masson's trichrome tissue staining, more collagen and unmineralized bone matrix were observed in the LLLT groups, and this result is supported by previous research results. In a histomorphometric study, the average BIC ratio values of the control group and the LLLT group at six weeks were 52.1%±18.3% and 55.4%±9.0%, respectively. The LLLT group showed increased values, but the results were not statistically significance. The average BIC ratio values of the control group and the LLLT group at 12 weeks were 50.4%±9.8% and 62.2%±10.2%, respectively. The LLLT group showed statistically significant higher values. This result indicates that LLLT treatment applied for one week after implantation stimulated bone formation. It also demonstrates that histopathological findings might not always match histomorphometric analysis results.
Histomorphometric exams have been considered the gold standard for accuracy in evaluating osseointegration of dental implants. However, tissue sections need to be prepared, and in vivo evaluation is not possible. In addition, this method is invasive, irreversible, and time-consuming12. Removal torque testing provides information about the degree of contact between a bone and an implant. Such studies have been performed in cadavers and experimental animals, and this method provides direct evidence of osseointegration, although it is also invasive and can cause irreversible transformation. Therefore, in clinical practice, radiologic methods are primarily used rather than the invasive methods mentioned above. Recently, studies have reported that dental implant stability can be evaluated using RFA, which is not invasive 1314. RFA was introduced by Meredith15 and has been found to be an effective method for evaluating implant stability. ISQ values derived from RFA range from 1 to 100 in general, and values from 60 to 65 are accepted as stable after the bone healing process1617.
In this research, the average ISQ values for the six-week groups were 68.3±15.5 and 69.0±10.0, respectively, and the average removal torque values were 60.0±32.5 cNm and 54.1±4.3 cNm. The control group had similar or higher values than the LLLT group, but the values were not statistically significant. For the 12-week groups, the average ISQ values were 73.3±6.6 and 65.7±9.7, respectively, and the average removal torque values were 37.4±6.1 cNm and 90.0±21.1 cNm. Here, the LLLT group had statistically significantly higher values.
The use of various evaluation methods that limit the number of samples for each method can affect the results, such as the observed different outcomes between LLLT and control groups of the six-week groups. However, in animal studies, the numbers of experimental animals are limited due to ethical concerns. Therefore, the use of various evaluation methods to ensure accuracy might produce practical limitations. Additionally, using experimental animals whose osseous tissues are similar to human maxillary bone is necessary for optimal assessment and comparison. Altogether, the results of this study are promising, although further in-depth research will be essential to determine whether LLLT has positive effects on osseointegration.
This research was conducted to analyze the effects of LLLT on osseointegration after placement of titanium implants in rabbit femurs and application of LLL. The LLLT had a positive effect on osseointegration at a cellular level. However, BIC ratio and ISQ do not match histopathological findings.
Notes
References
1. Kemmotsu O, Sato K, Furumido H, Harada K, Takigawa C, Kaseno S, et al. Efficacy of low reactive-level laser therapy for pain attenuation of postherpetic neuralgia. Laser Ther. 1991; 3:71–75.

2. Braverman B, McCarthy RJ, Ivankovich AD, Forde DE, Overfield M, Bapna MS. Effect of helium-neon and infrared laser irradiation on wound healing in rabbits. Lasers Surg Med. 1989; 9:50–58. PMID: 2927230.

3. Midamba E, Haanaes HR. Therapeutic effect of low level laser irradiation on inferior alveolar, mental and lingual nerve paresthesia. Laser Ther. 1993; 5:89–94.
4. Takeda Y. Irradiation effect of low-energy laser on alveolar bone after tooth extraction. Experimental study in rats. Int J Oral Maxillofac Surg. 1988; 17:388–391. PMID: 3145957.

5. Khadra M, Rønold HJ, Lyngstadaas SP, Ellingsen JE, Haanaes HR. Low-level laser therapy stimulates bone-implant interaction: an experimental study in rabbits. Clin Oral Implants Res. 2004; 15:325–332. PMID: 15142095.

6. Khadra M, Lyngstadaas SP, Haanaes HR, Mustafa K. Effect of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials. 2005; 26:3503–3509. PMID: 15621240.

7. Khadra M. The effect of low level laser irradiation on implant-tissue interaction: in vivo and in vitro studies. Swed Dent J Suppl. 2005; (172):1–63. PMID: 15906852.
8. Guzzardella GA, Torricelli P, Nicoli-aldini N, Giardino R. Osseointegration of endosseous ceramic implants after postoperative low-power laser stimulation: an in vivo comparative study. Clin Oral Implants Res. 2003; 14:226–232. PMID: 12656884.
9. Maluf AP, Maluf RP, Brito CR, França FM, De BRJ. Mechanical evaluation of the influence of low-level laser therapy in secondary stability of implants in mice shinbones. Lasers Med Sci. 2010; 25:693–698. PMID: 20393769.

10. Kim YD, Kim SS, Hwang DS, Kim SG, Kwon YH, Shin SH, et al. Effect of low-level laser treatment after installation of dental titanium implant-immunohistochemical study of RANKL, RANK, OPG: an experimental study in rats. Lasers Surg Med. 2007; 39:441–450. PMID: 17523169.

11. Pereira CL, Sallum EA, Nociti FH Jr, Moreira RW. The effect of low-intensity laser therapy on bone healing around titanium implants: a histometric study in rabbits. Int J Oral Maxillofac Implants. 2009; 24:47–51. PMID: 19344024.
12. Becker W, Sennerby L, Bedrossian E, Becker BE, Lucchini JP. Implant stability measurements for implants placed at the time of extraction: a cohort, prospective clinical trial. J Periodontol. 2005; 76:391–397. PMID: 15857073.

13. Friberg B, Sennerby L, Linden B, Gröndahl K, Lekholm U. Stability measurements of one-stage Brånemark implants during healing in mandibles: a clinical resonance frequency analysis study. Int J Oral Maxillofac Surg. 1999; 28:266–272. PMID: 10416893.
14. Oh JS, Kim SG. Clinical study of the relationship between implant stability measurements using Periotest and Osstell mentor and bone quality assessment. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 113:e35–e40. PMID: 22669155.

15. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998; 11:491–501. PMID: 9922740.
16. Atsumi M, Park SH, Wang HL. Methods used to assess implant stability: current status. Int J Oral Maxillofac Implants. 2007; 22:743–754. PMID: 17974108.
17. Glauser R, Sennerby L, Meredith N, Rée A, Lundgren A, Gottlow J, et al. Resonance frequency analysis of implants subjected to immediate or early functional occlusal loading: successful vs. failing implants. Clin Oral Implants Res. 2004; 15:428–434. PMID: 15248877.

Fig. 1
Differences between old lamellar bone, at a distance >1 mm from the implant surface, and new bone (arrowheads) formation all along the implant surface. Microphotograph at 6 weeks after implantation (H&E staining, ×100) in the control group (A) and the low-level laser therapy (LLLT) group (B). Microphotograph at 12 weeks after implantation (H&E staining, ×100) in the control group (C) and the LLLT group (D).
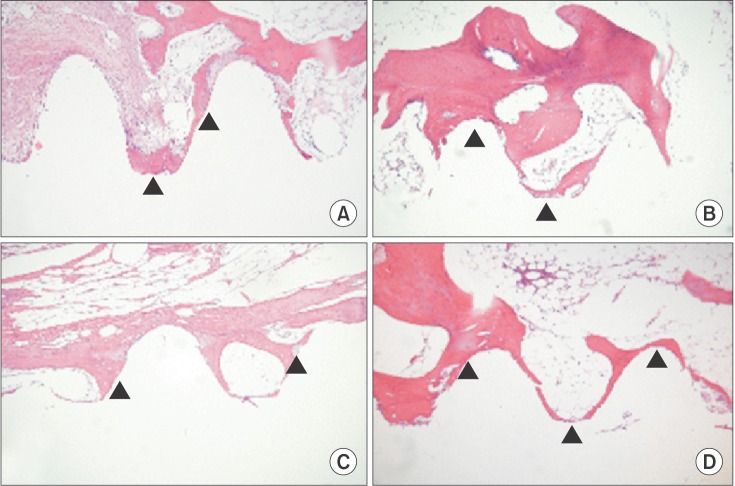
Fig. 2
Differences between old lamellar bone, at a distance >1 mm from the implant surface, and new bone (arrowheads) formation along the implant surface. Microphotograph at 6 weeks after implantation (Masson's trichrome staining, ×100) in the control group (A) and the low-level laser therapy (LLLT) group (B). Microphotograph at 12 weeks after implantation (Masson's trichrome staining, ×100) in the control group (C) and the LLLT group (D).
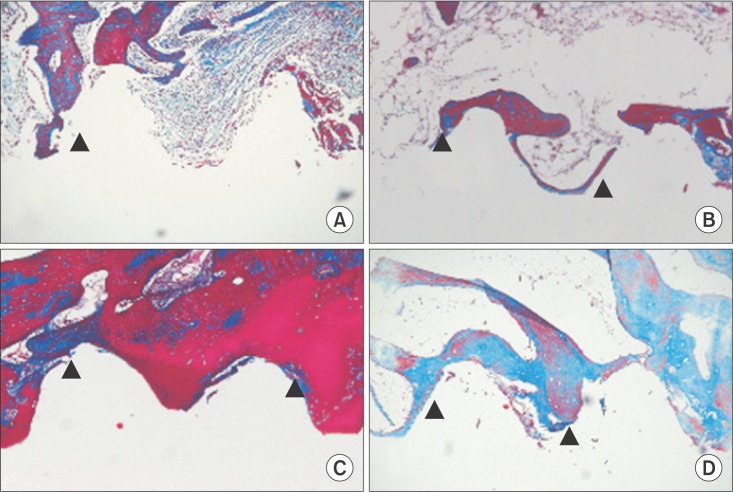
Fig. 3
Mean bone-to-implant contact (BIC) ratio in the control group and the low-level laser therapy (LLLT) group.

Table 1
Descriptive statistics of removal torque value according to two groups and healing time
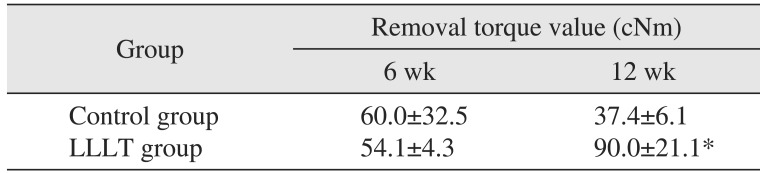
| Group | Removal torque value (cNm) | |
|---|---|---|
| 6 wk | 12 wk | |
| Control group | 60.0±32.5 | 37.4±6.1 |
| LLLT group | 54.1±4.3 | 90.0±21.1* |
Table 2
Descriptive statistics of ISQ, dif. % ISQ according to two groups and healing time
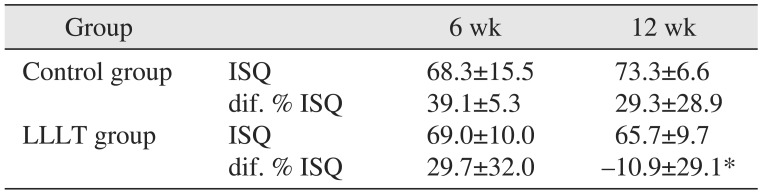
| Group | 6 wk | 12 wk | |
|---|---|---|---|
| Control group | ISQ | 68.3±15.5 | 73.3±6.6 |
| dif. % ISQ | 39.1±5.3 | 29.3±28.9 | |
| LLLT group | ISQ | 69.0±10.0 | 65.7±9.7 |
| dif. % ISQ | 29.7±32.0 | -10.9±29.1* |
(ISQ: implant stability quotient, dif. % ISQ: converted into a percentage for the difference values between final ISQ and initial ISQ, LLLT: low-level laser therapy)
*P≤0.05.
LLLT group was treated with LLLT for one week after the surgery and control group was not treated with LLLT.
ISQ, dif. % ISQ in the control group and LLLT group at different time intervals postsurgery.
Values are presented as mean±standard deviation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download