Abstract
Oral mucormycosis is a fungal infection observed mainly in elderly immunocompromised patients. In rare instances, the disease occurs in healthy individuals and those patients that are below preschool age. Although this condition mainly involves the maxilla, it may also manifest in any part of the oral cavity based on the source of infection. Mucormycosis of the maxilla spreads rapidly, leading to necrosis of the palatal bone and palatal perforation. Such patients are usually rehabilitated using bone grafting or free flap surgeries. However, when surgeries are delayed, palatal prosthesis is an interim treatment modality that can prevent nasal regurgitation and aspiration of food or fluids. Palatal prostheses also help with mastication, speech, and swallowing. The present case describes a rare case of oral mucormycosis in an 18-month-old male involving the maxilla that was managed by palatal prosthesis.
Go to : 
Mucormycosis is an acute opportunistic infection usually seen in immunocompromised patients, particularly those with uncontrolled diabetes and leukemia12. Paltauf3 in 1885 was the first to describe mucormycosis. The disease is caused by a saprophytic fungus, mainly Mucor or Rhizopus , which is considered the most deadly and rapidly progressive form of fungal infection in humans4. Although decaying fruits and vegetables are the main sources of fungal infection, spores of fungi can also be inhaled from dust or air from air conditioners affecting paranasal sinuses567. Patients may also develop cellulites, fever, headache, necrotic turbinates, and nasal discharge. If left untreated the diseases may spread into the brain resulting in death6. Healthy individuals are rarely affected by mucormycosis and those affected have predisposing risk factors such as a history of tooth extraction, pneumonia, severe burns, and gastrointestinal or rhinocerebral infections. Mucormycosis is generally observed after the third decade of life891011. Until now there have been no cases reported in patients below the age of five years. The present case report outlines the classical oral manifestations and dental management of mucormycosis in an 18-month-old male.
Go to : 
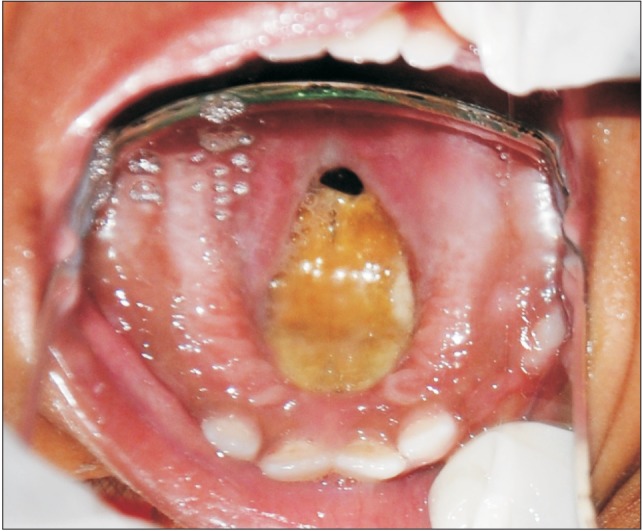
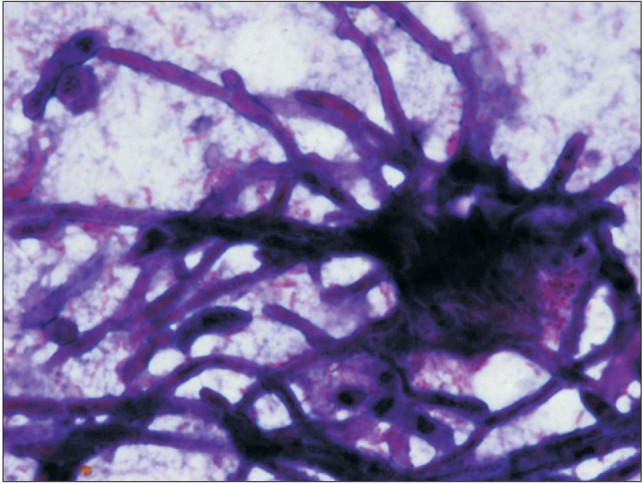
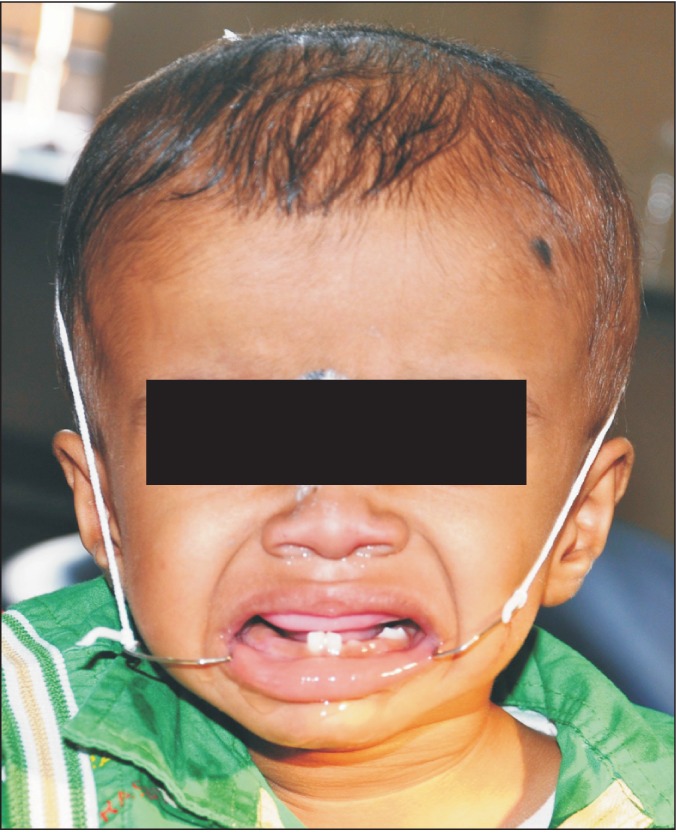
An 18-month-old boy reported to our department with a complaint of extensive ulcer on the hard palate for three days, nasal regurgitation of food, and a foul smell. History reveled pneumonia one month prior that was treated in a medical hospital. After one month, the family of the patient noticed a small palatal ulcer for which he was again hospitalized. Within three days the palatal ulcer increased in size and lead to palatal perforation. The patient experienced difficulty in swallowing and nasal regurgitation. Thereafter the child was referred to our department for palatal prosthesis to prevent nasal regurgitation and aspiration pneumonia. The past medical history was not significant, particularly for uncontrolled juvenile diabetics or hematologic malignant conditions. There was no history of sinusitis or nasal discharge. Intraoral examination revealed an extensive palatal ulcer with exposed bone that extended from the mid palatine raphe to the soft palate, leading to palatal perforation.(Fig. 1) The floor of the mouth, tongue, buccal mucosa, lips, and nasal septum were normal. The primary maxillary central and lateral incisors, primary maxillary left first molar, primary mandibular central incisors, and primary mandibular left first molar were partially erupted.(Fig. 1) An occlusal radiograph of the maxilla showed a diffuse radiolucency of approximately 3×4 cm2 in size in the mid-palatine region. Cortical outline of the crypt of permanent maxillary central incisors were lost. The radiograph also showed a change in position of the tooth bud of the permanent maxillary central incisors.(Fig. 2) A probable diagnosis of mucormycosis was made. Fragments of palatal soft tissue were obtained for histological examination. The histological examination showed a broad, non-septate fungal hyphae with obtuse angle branching and necrotic blood vessels suggestive of mucormycosis.(Fig. 3) Thereafter, the patient was referred to a medical hospital for treatment of mucormycosis. Intravenous amphotericin (5.5 mg) was given to the patient for 31 days. During this period, a nasogastric tube was used to feed the patient. After one month the patient was again referred to our department for palatal prosthesis. The overall physical health of the patient was weak as he was unable to eat. To facilitate swallowing and to prevent aspiration pneumonia palatal prosthesis was advised. Maxillary jaw impression was made using irreversible hydrochloride (Zelgam; Dentsply DeTrey GmbH, Konstanz, Germany). To prevent aspiration of irreversible hydrochloride (palatal perforation), a thin piece of gauge (2×2 cm2) was placed over the mixed irreversible hydrochloride. The impression was poured in Gypsum II material (dental plaster; Kalabhai Karson, Mumbai, India). A special tray was fabricated using cold cure resin (DPI RR Cold Cure; Dental Products of India, Mumbai, India) and the final impression was made using medium body polyvinyl siloxane elastomer (Examix NDS; GC America Inc., Alsip, IL, USA) and poured with Gypsum II material to obtain the final cast. In the area of erupting teeth, a spacer of 2 mm was given. Next, a palatal prosthesis was fabricated using heat cured acrylic resin (Lucitone 99; Dentsply International, York, PA, USA). Thereafter, a 19-gauge wire with right angle bends as shown in Fig. 4, 5 was acralized to the palatal prosthesis using cold cure acrylic resin to avoid trauma to the commissures. As there were no fully erupted teeth, retention of palatal prosthesis was obtained using elastic thread tied to the 19-gauge stainless steel wire.(Fig. 4, 5) Initially, the child was asked to wear the palatal prosthesis for 30 minutes at an interval of 2 hours for a week. After a week, the patient was advised to begin a liquid and semi-solid diet. After 15 days, the child was comfortable with the palatal prosthesis and was able to eat properly.(Fig. 6) There was neither regurgitation nor aspiration of food from the palatal perforation. As the child was not fit for general anesthesia, a surgical procedure was postponed. The patient was evaluated every month to assess the palatal prosthesis in terms of erupting teeth. After three months, the patient once again developed foul smell from the infected area. He was referred to a medical hospital for further treatment. Follow-up visits were planned for trimming palatal prosthesis to facilitate eruption of teeth.
Go to : 
Inhalation of fungi spores or direct wound contamination are the two most common causes for oral mucormycosis. Palatal mucormycosis is usually observed when the infection spreads through the nose and paranasal sinuses. When wound contamination is the source of infection, it can manifest in any part of the oral cavity12. Although mucormycosis of the maxilla is more common, it is difficult to predict the source of infection because saprophytic fungus can be cultured from the oral cavity of healthy humans and it is possible that these opportunistic organisms can be implanted into the maxilla during any oral surgical procedure, such as extraction, periodontal surgeries, and abscess drainage5. In such circumstances, a history of a recent surgical procedure can be used as a diagnostic tool to determine the cause of infection. In the present case, inhalation was the source of infection because there was no history of oral surgical procedure, although the patient had a past hospitalization for pneumonia. There may have been spread of infection through the nasal cavity or sinuses, leading to maxilla involvement via blood. This occurred mainly through the internal maxillary artery or descending palatine artery by thromboembolic processes413. These opportunistic organisms have an affinity for invading blood vessel walls by direct extension and produce vascular thrombosis1415. Within no time, the tissue supplied by the thrombous vessel undergoes widespread tissue necrosis, exposing the underlying bone413.
The majority cases of oral mucormycosis have been found in the fourth to sixth decades of life891011. This is because oral mucormycosis mainly target immunocompromised patients such as those with uncontrolled diabetic, leukemia, lymphoma, and AIDS12. The same is not always true because healthy humans are also, but rarely, affected by mucormycosis; this might be due to exposure to various predisposing risk factors. The risk factors could be systemic (burns, protein energy malnutrition, pneumonia) or local (teeth extraction, periodontal surgeries). In the literature, various cases of oral mucormycosis have been reported. Huang et al.16 reported a 40-year-old patient with oral mucormycosis and extensive maxillary osteonecrosis secondary to dental extraction. Fogarty et al.17 reported a 74-year-old male patient with chronic obstructive pulmonary disease who developed mucormycosis of the maxilla following dental extraction. Kumar et al.18 reported mucormycosis of the maxilla in a 65-year-old diabetic and immune-compromised male patient managed with a removable prosthesis. There are other reports of two cases by Bakathir19, in which one case was a 14-year-old male patient with oral mucormycosis of the maxilla and mandible undergoing chemotherapy for acute myeloid leukemia. The second case involved the mandible of a 49-year-old male patient recently diagnosed with type 2 diabetic mellitus with ketoacidosis and underlying undiagnosed acute lymphoblastic leukemia. To the best of our knowledge, this case represents the first report of oral mucormycosis in a healthy 18-monthold child with a predisposing risk factor of pneumonia.
Oronasal infections can be life threatening in children. Food or fluids can enter the sinuses or lungs and predispose children to sinusitis, middle ear infection, pneumonia, and ultimately death. Surgical closure should be the treatment of choice for oronasal communication. However, when children are not fit for surgery, palatal prosthesis is the choice of treatment. Palatal prosthesis helps with chewing, swallowing, mastication, phonetics, and esthetics, and also prevents regurgitation or aspiration of food or fluids2021. Shah et al.22 reported a case of a 42-year-old female with an acquired maxillary defect as a result of surgical treatment of mucormycosis. The palatal prosthesis given to this patient not only helped with chewing and swallowing but also assisted in improving phonetics and esthetics. Gowda et al.23 reported a case of maxillary mucormycosis in which the implant supported a magnetic retentive prosthesis that was given to the patient. The implant supported magnetic unit offered a practical method of improving retention. In the present case, retention of the palatal prosthesis was complicated by two reasons: i) missing posterior teeth and ii) compliance from the child, as he was 18 months old. Therefore, elastics were used to hold the palatal prosthesis in place during mastication. Prosthetic rehabilitation of oronasal fistula offers several advantages, such as opportunity for immediate dental restoration without the need for further surgery and its cost effectiveness. However, a study conducted by Genden et al.24 documents that mastication, speech, oronasal refluxes, and functional quality of life was superior in patients rehabilitated with vascularized bonecontaining free flap than with palatal prosthesis. Rehabilitation of the oronasal fistula using palatal prosthesis should not be the replacement for reconstructive surgeries. However, it can be recommended as an interim treatment option until patients are fit for reconstructive surgeries.
Thromboembolic events compromise the blood supply to the affected area, resulting in necrosis. Rapid necrosis of the maxilla can be either due to infection or may be iatrogenic in nature. The most common oral infections leading to necrosis of bone are bacterial, followed by viral (herpes zoster) and fungal; whereas radiation and trauma are iatrogenic causes2526. While unlikely in the setting of fungal infections, herpes zoster infection usually presents with a painful rash with vesicular eruptions along the sensory nerve resulting in facial nerve palsy representing Ramsey Hunt syndrome. More importantly, in herpes zoster infections the teeth rapidly exfoliate, following osteonecrosis in a dermatomal pattern that does not cross the midline. Secondly, patients with herpes zoster would have past history of exposure to chicken pox, which is absent in a patient with oral mucormycosis252627. While immunity after chickenpox infection is thought to be lifelong, a study by Weatherall et al.28 showed that antibodies may not be detected after a particular age, which may be a reason why reactivation of herpes zoster usually occurs in older patients. The exact mechanism for bone necrosis in herpes zoster is unknown. It is assumed that sympathetic nerves that accompany the vasculature cause intense vasoconstriction, leading to ischemia and necrosis of the maxillary bone2930. Bacterial infections may cause rapid necrosis and osteomyelitis, leading to rapid necrosis of bone resembling mucormycosis. However, in bacterial infection, patients usually show erythema, abscess, swelling, pain, discomfort, and loss of function of the affected part, which are rarely observed in mucormycosis31. Actinomycosis of the oral cavity is very rare in humans. If it occurs, the tissue surrounding the affected/necrosed bone is swollen, while erythematous and micro abscesses show discharge of sulphur granules3233. These observations are not pathognomic of mucormycosis. In the present case, an iatrogenic cause was ruled out because the patient did not have a history of radiation therapy and did not have a history of recent trauma such as dental extraction.
Histologic specimens of the necrotic wound surface can diagnose mucormycosis, but the final diagnosis must always be confirmed using molecular pathologic examination. There is a close histopathological resemblance between mucormycosis and aspergilliosis. Microscopically, aspergilliosis has septa branching hyphae that can be distinguished from mucormycotic hyphae by a smaller width and prominent acute angulations of branching hyphae34.
Despite early treatment, the mortality rate of patients with mucormycosis is very high, ranging from 16% to 100%. Cutaneous mucormycosis has a mortality rate of 17%, whereas rhinocerebral, pulmonary, and gastrointestinal mucormycosis have mortality rates of 67%, 83%, and 100%, respectively. However, early medical (systemic administration of amphotericin B) and surgical (radical surgical intervention) treatment limits the spread of fungal infection to vital regions of the body and improves patient survival by 80%35.
The present case is the youngest patient reported with mucormycosis. A feeding plate was given to the patient to prevent food regurgitation. However, as multiple teeth were missing, intraoral retention of the feeding plate was a major problem. This was overcome by using extraoral retention.
In conclusion, palatal prostheses can be an interim treatment modality to prevent nasal regurgitation and aspiration of food or fluids in patients with palatal perforation. It also assists in swallowing, mastication, and phonetics until the final treatment is provided to the patient.
Go to : 
References
1. Rosenberg SW, Lepley JB. Mucormycosis in leukemia. Oral Surg Oral Med Oral Pathol. 1982; 54:26–32. PMID: 6956824.

2. Abramson E, Wilson D, Arky RA. Rhinocerebral phycomycosis in association with diabetic ketoacidosis. Report of two cases and a review of clinical and experimental experience with amphotericin B therapy. Ann Intern Med. 1967; 66:735–742. PMID: 4960691.
4. Lehrer RI, Howard DH, Sypherd PS, Edwards JE, Segal GP, Winston DJ. Mucormycosis. Ann Intern Med. 1980; 93:93–108. PMID: 6772062.

5. Taylor CG, Alexander RE, Green WH, Kramer HS Jr. Mucormycosis (phycomycosis) involving the maxilla. Report of a case with survival. Oral Surg Oral Med Oral Pathol. 1969; 27:806–822. PMID: 5254571.
6. Jones AC, Bentsen TY, Freedman PD. Mucormycosis of the oral cavity. Oral Surg Oral Med Oral Pathol. 1993; 75:455–460. PMID: 8464609.

7. Weprin BE, Hall WA, Goodman J, Adams GL. Long-term survival in rhinocerebral mucormycosis. Case report. J Neurosurg. 1998; 88:570–575. PMID: 9488314.
8. Bonifaz A, Macias B, Paredes-Farrera F, Arias P, Ponce RM, Araiza J. Palatal zygomycosis: experience of 21 cases. Oral Dis. 2008; 14:569–574. PMID: 18248590.

9. Deboni MC, Pozzani VR, Lisboa T, Hiraki K, Viplich R, Naclério-Homem MG. Mucormycosis in an immunocompetent patient: follow-up of 1 year after treatment. Acta Otolaryngol. 2006; 126:993–996. PMID: 16864500.

10. Aras MH, Kara MI, Erkiliç S, Ay S. Mandibular mucormycosis in immunocompromised patients: report of 2 cases and review of the literature. J Oral Maxillofac Surg. 2012; 70:1362–1368. PMID: 21820787.

11. Samanta DR, Senapati SN, Sharma PK, Shruthi BS, Paty PB, Sarangi G. Hard palate perforation in acute lymphoblastic leukemia due to mucormycosis: a case report. Indian J Hematol Blood Transfus. 2009; 25:36–39. PMID: 23100971.
12. McNulty JS. Rhinocerebral mucormycosis: predisposing factors. Laryngoscope. 1982; 92:1140–1143. PMID: 7132514.
14. Leitner C, Hoffmann J, Zerfowski M, Reinert S. Mucormycosis: necrotizing soft tissue lesion of the face. J Oral Maxillofac Surg. 2003; 61:1354–1358. PMID: 14613095.

15. Pogrel MA, Miller CE. A case of maxillary necrosis. J Oral Maxillofac Surg. 2003; 61:489–493. PMID: 12684969.

16. Huang JS, Kok SH, Lee JJ, Hsu WY, Chiang CP, Kuo YS. Extensive maxillary sequestration resulting from mucormycosis. Br J Oral Maxillofac Surg. 2005; 43:532–534. PMID: 16024140.

17. Fogarty C, Regennitter F, Viozzi CF. Invasive fungal infection of the maxilla following dental extractions in a patient with chronic obstructive pulmonary disease. J Can Dent Assoc. 2006; 72:149–152. PMID: 16545177.
18. Kumar JA, Babu P, Prabu K, Kumar P. Mucormycosis in maxilla: Rehabilitation of facial defects using interim removable prostheses: a clinical case report. J Pharm Bioallied Sci. 2013; 5(Suppl 2):S163–S165. PMID: 23956598.

19. Bakathir AA. Mucormycosis of the jaw after dental extractions: two case reports. Sultan Qaboos Univ Med J. 2006; 6:77–82. PMID: 21748139.
20. Zlotolow IM. Dental oncology and maxillofacial prosthtetics. In : Shah JP, editor. Cancer of head and neck: atlas of clinical oncology. Hamilton: BC Decker Inc.;2001. p. 373–376.
21. Tanaka H. Speech patterns of edentulous patients and morphology of the palate in relation to phonetics. J Prosthet Dent. 1973; 29:16–28. PMID: 4508620.

22. Shah RJ, Katyayan MK, Katyayan PA, Chauhan V. Prosthetic rehabilitation of acquired maxillary defects secondary to mucormycosis: clinical cases. J Contemp Dent Pract. 2014; 15:242–249. PMID: 25095851.

23. Gowda ME, Mohan MS, Verma K, Roy ID. Implant rehabilitation of partial maxillectomy edentulous patient. Contemp Clin Dent. 2013; 4:393–396. PMID: 24124314.
24. Genden EM, Okay D, Stepp MT, Rezaee RP, Mojica JS, Buchbinder D, et al. Comparison of functional and quality-of-life outcomes in patients with and without palatomaxillary reconstruction: a preliminary report. Arch Otolaryngol Head Neck Surg. 2003; 129:775–780. PMID: 12874081.
25. Owotade FJ, Ugboko VI, Kolude B. Herpes zoster infection of the maxilla: case report. J Oral Maxillofac Surg. 1999; 57:1249–1251. PMID: 10513873.

26. Hall HD, Jacobs JS, O'Malley JP. Necrosis of maxilla in patient with herpes zoster. Report of a case. Oral Surg Oral Med Oral Pathol. 1974; 37:657–662. PMID: 4524373.
27. Barrett AP, Katelaris CH, Morris JG, Schifter M. Zoster sine herpete of the trigeminal nerve. Oral Surg Oral Med Oral Pathol. 1993; 75:173–175. PMID: 8381216.

28. Weatherall DJ, Ledingham JGG, Warrell DA. Oxford teatbook of medicine. 3rd ed. Oxford: Oxford University Press;1996. p. 197–221.
29. Mintz SM, Anavi Y. Maxillary osteomyelitis and spontaneous tooth exfoliation after herpes zoster. Oral Surg Oral Med Oral Pathol. 1992; 73:664–666. PMID: 1437032.

30. Cooper JC. Tooth exfoliation and osteonecrosis of the jaw following herpes zoster. Br Dent J. 1977; 143:297–300. PMID: 270353.

31. Nandakumar H, Shankaramba KB. Massive sequestration of the upper jaw: a case report. Br J Oral Maxillofac Surg. 1990; 28:55–56. PMID: 2182111.

32. Garg R, Schalch P, Pepper JP, Nguyen QA. Osteomyelitis of the hard palate secondary to actinomycosis: a case report. Ear Nose Throat J. 2011; 90:E11–E12. PMID: 21412725.

33. Finley AM, Beeson MS. Actinomycosis osteomylelitis of the mandible. Am J Emerg Med. 2010; 28:118.e1–118.e4. PMID: 20006231.

34. Manjunatha BS, Das N, Sutariya RV, Ahmed T. Mucormycosis of the hard palate masquerading as carcinoma. Clin Pract. 2012; 2:e28. PMID: 24765427.

35. Verma A, Singh V, Jindal N, Yadav S. Necrosis of maxilla, nasal, and frontal bone secondary to extensive rhino-cerebral mucormycosis. Natl J Maxillofac Surg. 2013; 4:249–251. PMID: 24665188.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download