I. Introduction
In 1952, Wilson
1 coined the term "necrotizing fasciitis" (NF) for the necrotizing soft tissue infections in which the fascia and tissue death are invariably involved. NF is an infection that spreads along the fascial planes causing subcutaneous tissue death with relative sparing of skin and underlying muscles, characterized by rapid progression, systemic toxicity, and even death
2. Histologically, obliterative vascular thrombosis and polymorphonuclear infiltration are initially confined to the deep dermis and superficial fascia
3. Liquefactive necrosis of subcutaneous fat, air tracking along deep fascial planes, and vascular thrombosis with resultant skin changes eventually occur.
The portal of bacterial entry may be trivial, and patients usually present with pain disproportionate to the visible skin changes
2. Presenting symptoms are nonspecific and may include mild cellulitis, edema, and occasionally crepitation
2.
At onset, NF can be difficult to differentiate from cellulitis and other superficial infections of the skin
4. Studies have shown that only 15% to 34% of patients with NF have an accurate admitting diagnosis
5. Patients are sometimes treated for simple cellulitis until they rapidly deteriorate.
Patients with NF usually demonstrate systemic effects, initially presenting with fever (temperature greater than 38℃), tachycardia, diaphoresis, and possibly even an altered mental state or diabetic ketoacidosis. The physical examination should include all parts of the body in a search for skin inflammation. This is especially necessary for patients who present with sepsis of unknown origin. The perineum and oral cavity are areas that can be easily missed
4.
Misdiagnosis and delayed treatment can result in death from sepsis, mediastinitis, carotid artery erosion, jugular vein thrombophlebitis, or aspiration pneumonia
6. NF is associated with systemic toxicity and high mortality rate if not treated timely and aggressively.
Our department experienced an NF patient who showed severe skin infection around the neck and required several operations. Herein, we share our clinical experience of diagnosis and treatment of NF.
Go to :

II. Case Report
A 85-year-old female patient visited our emergency room due to painful swelling on the bilateral submandibular, submental, and upper neck areas. The overlying skin was edematous and reddish (
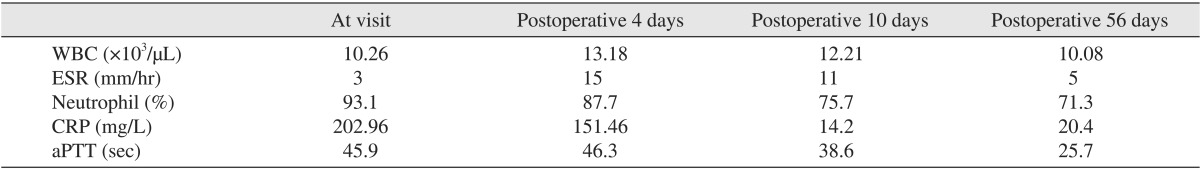
Fig. 1), with an ill-defined reddish border. She complained of extreme pain in the upper neck area. Several days previous, she underwent endodontic treatment on a lower central incisor. After the treatment, however, her symptoms persisted, and swelling developed suddenly. She was febrile, with a body temperature of 39℃. No other medical condition was significant except a medical history of steroid due to arthralgia. Laboratory findings were a white blood cell (WBC) of 10.26×10
3/µL, erythrocyte sedimentation rate (ESR) of 3 mm/hr, neutrophil of 93.1%, activated partial thromboplastin time (aPTT) of 45.9 seconds, and a C-reactive protein (CRP) of 202.96 mg/L (
Table 1), suggesting an acute infected state. Comuted tomography (CT) was performed and showed a disseminated deep neck abscess in the left submandibular space that extended into the upper mediastinum through the anterior neck space and to the supraclavicular and axillary areas.(
Fig. 2)
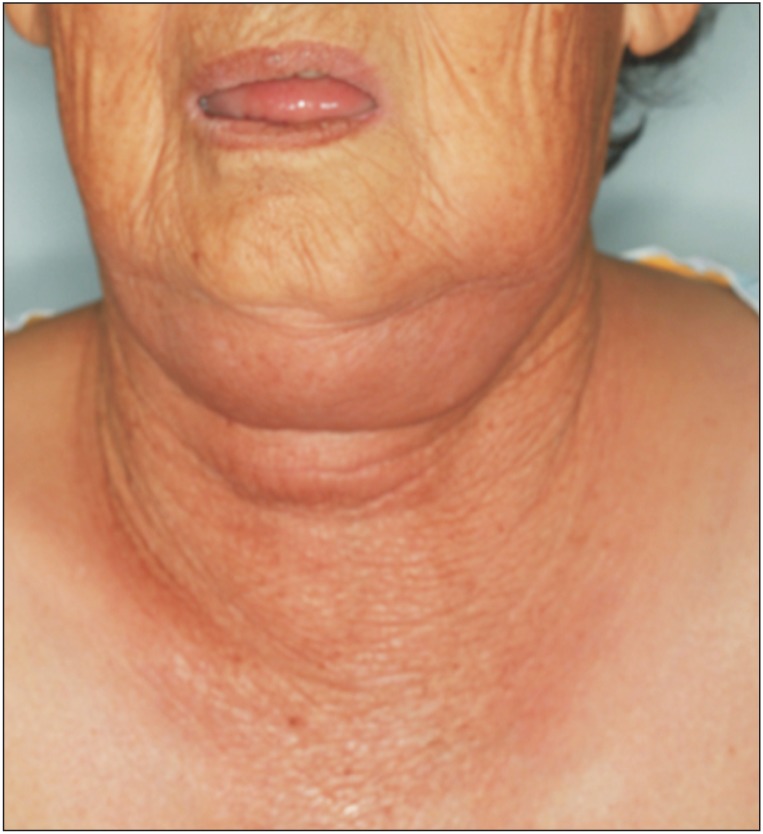 | Fig. 1On clinical examination, the patient showed swollen and reddish skin in the submandibular, submental, and upper neck areas.
|
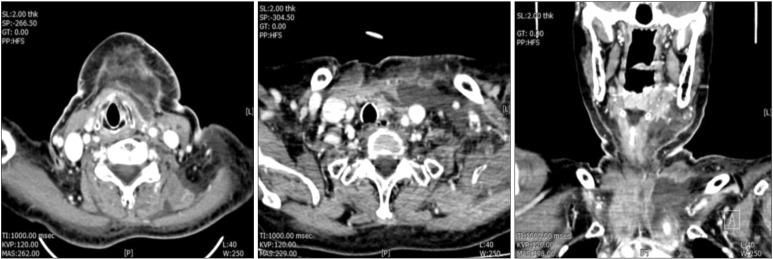 | Fig. 2Computed tomographies showed a disseminated deep neck space abscess in the left submandibular space that extended into the upper mediastinum through the anterior neck space and to the supraclavicular and axillary areas.
|
Table 1
Laboratory findings during the disease process

Initial diagnosis was odontogenic abscess. We promptly drained the abscess (
Fig. 3) by incising the submental and submandibular areas and dissecting with fingers and mesenbaum scissors. When the underlying fascia was opened, a small amount of pus was discharged, and the fascia was noted to be a discolored grayish color. These findings suggested fasciitis rather than odontogenic abscess. To drain the anterior neck and superior mediastinum, we incised the skin along the anterior border of the sternocleidomastoid muscle, revealing that that entire fascia along the anterior neck and carotid sheath was discolored to grayish. With a scissors and fingers, we detached the diseased fascia from the submental area to the upper mediastinum. To drain the supraclavicular and axillary areas, we incised the supraclavicular area and the anterior border of the trapezius muscle, and the infected areas were explored. Silicon drains were inserted, and the patient was administered a triple antibiotic regimen of penicillin, aminoglycoside, and metronidazole. On microscopic examination, acute and chronic infection was found, and lymphocyte infiltrations were noted in the necrotic fascia. We diagnosed this condition as NF. Pus culture results showed betahemolytic streptococcal infection, a microorganism sensitive to most antibiotics. Based on this, we continued the triple antibiotic regimen.
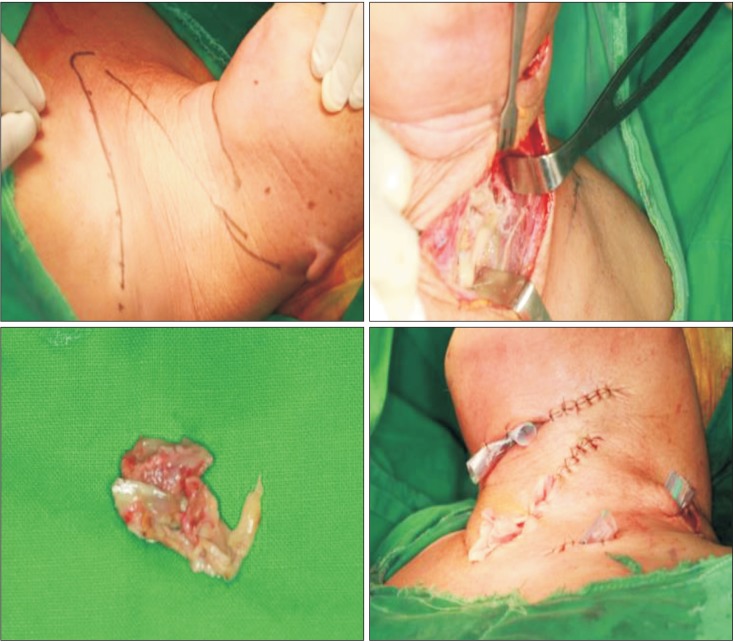 | Fig. 3Necrotic fascia was removed, and the infected area were drained. The fascia was fragile and discolored to grayish.
|
After 4 days, the patient's condition was not improved, and CT showed that the infection had spread under the trapezius muscle. The skin over the shoulder area was intense hot and reddish. Airway obstruction was expected if the infection did not subside. Laboratory findings were WBC of 13.18×10
3/µL, ESR of 15 mm/hr, neutrophil of 87.7%, aPTT of 46.3 seconds, and a CRP of 151.46 mg/L.(
Table 1) We drained the area under the trapezius muscle and obtained an artificial airway through tracheostomy.(
Fig. 4) We incised the anterior border of the trapezius muscle to a greater extent than in the original surgery, incised the suprascapular area, and inserted the drain through and through.(
Fig. 4) We also changed the old drain. After the operation, the patient was sent to the intensive care unit and and administered ventilator therapy for respiratory support.
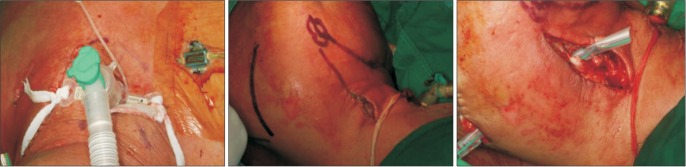 | Fig. 4An artificial airway was secured, and the area under the trapezius muscle was drained.
|
After 10 days, the skin infection had extended to the upper chest and shoulder area. Follow-up CT images showed that the multifocal abscess around the left chest wall, shoulder area and carotid area had further deteriorated.(
Fig. 5) Laboratory findings were a WBC of 12.21×10
3/µL ESR of 11 mm/hr, neutrophil of 75.7%, aPTT of 38.6 seconds, and a CRP of 14.2 mg/L.(
Table 1) We suspected uncontrolled NF, as there was no significant gross interval change in the multispace abscess or NF. We immediately performed a third operation in which we incised the skin on the chest over the healthy area and dissected the infected skin. Once the skin was elevated, we noted that the subcutaneous fat and superficial fascia were all necrotized. With finger dissection, the carotid space was dissected; all necrotic tissue around the upper chest and trapezius area was dissected with messenbaum scissors. Pus culture at this operation showed that the main microorganism was Actinibacter baumanni , which is resistant to cabepenem and most antibiotics. Based on this knowledge, we changed the triple antibiotic regimen to vancomycin monotherapy.
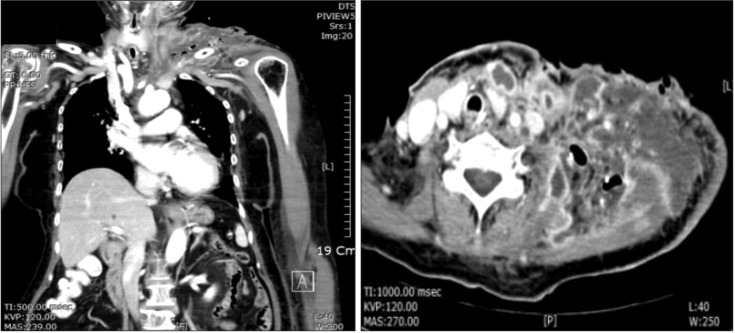 | Fig. 5Deterioration of the multifocal abscess around the left chest wall, shoulder area, and carotid area.
|
After fasciotomy, the skin was loosely sutured, and drains were inserted.(
Fig. 6) Fortunately, the infection decreased gradually after fasciotomy.(
Fig. 7)
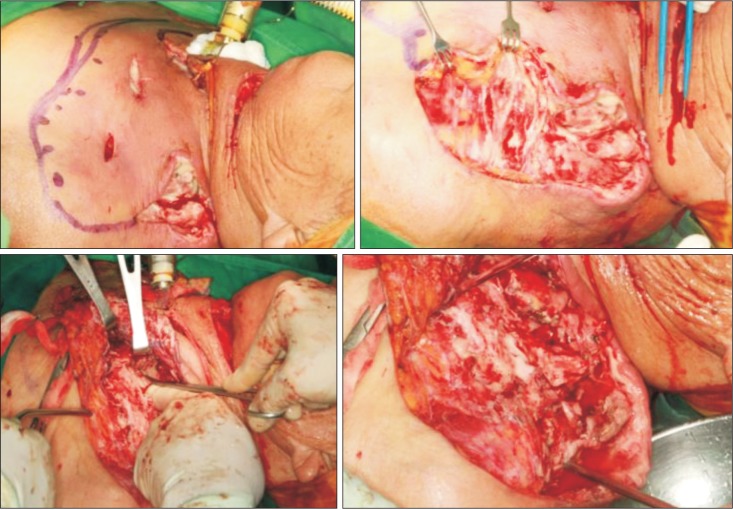 | Fig. 6After skin elevation, all necrotic fascia were exposed. We performed fasciotomy with fingers and scissors around the upper chest and carotid and shoulder areas.
|
 | Fig. 7Markedly decreased infection state.
|
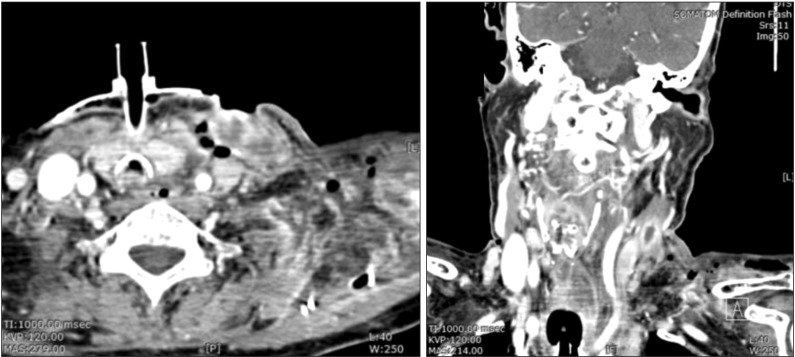
Although the infection subsided after the third operation, the skin over the distal clavicular area was not able to be salvaged. Using a bilobed rotational flap, the skin was closed on postoperative 37 days.(
Fig. 8) The tracheostomy tube was removed on postoperative 40 days. The patient was discharged on postoperative 56 days in good condition. Laboaratory findings on the day of discharge were a WBC of 10.08×10
3/µL ESR of 5 mm/hr, neutrophil of 71.3%, aPTT of 25.7 seconds, and a CRP of 20.4 mg/L.(
Table 1) The WBC was not high, but CRP and aPTT were extremely high, suggesting a recovery pattern related to reduced CRP and aPTT.
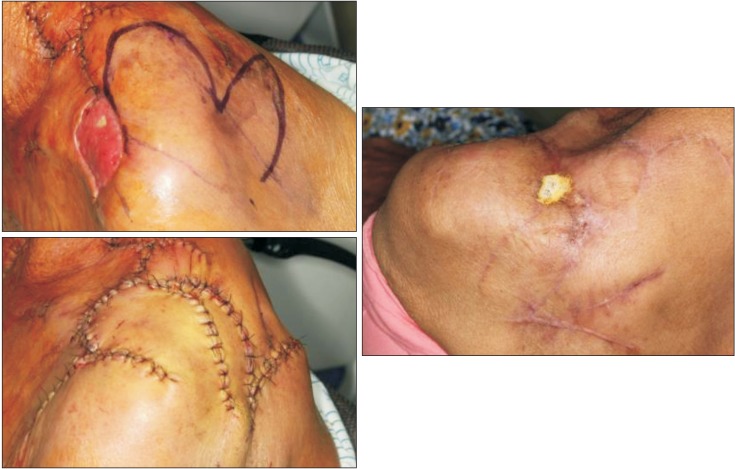 | Fig. 8After the infection subsided, the shoulder skin was not salvagable. The defect was closed with bilobed
rotational flap.
|
Go to :

III. Discussion
It is generally accepted that predisposing factors to NF include diabetes, alcoholism, tobacco abuse, immunosuppression, malnutrition, advanced age, peripheral vascular disease, renal failure, underlying malignancy, and obesity
7. Precipitating events of NF include surgery, minor invasive procedures (e.g., joint aspiration and acupuncture), intravenous drug use, penetrating injuries (e.g., insect and animal bites), soft tissue infection, burns, and child birth
4.
Most cases of NF occur in the abdomen, groin, and extremities. NF in the head and neck region is rare, with most cases having an odontogenic origin
7. Other causes of NF reported in the literature are tonsillar or pharyngeal infections, trauma, cervical adenitis, salivary gland infections, and tumor infection
8,
9. Cervicofacial NF is rarely initially suspected because the disease is uncommon, the presenting signs and symptoms are often benign, and there is frequently a dental infection or pharyngitis thought to explain the findings
6. In the presented case, patient had been administered corticosteroid due to arthralgia, which reflected an immunocompromised state, and the infection was induced by minor endodontic treatment. The patient was initially diagnosed with odontogenic infection, but necrotic fascia confirmed fasciitis.
According to Quereshy et al.
7, oral streptococcal species are cultured from most NF, with staphylococcal and anaerobic organisms also occasionally identified. These infections appear to be caused by the same species of bacteria that cause chronic dental subapical infections, acute localized cellulitis, and tissue abscess. The present case demonstrated a culture result of beta hemolytic streptococcal infection.
The classical infectious NF process usually begins 2 to 4 days after the initial insult. True NF features rapid, progressive liquefaction of the subcutaneous fat and connective tissue, while the overlying skin is spared. Necrosis and liquefaction of fascia and fat occur early in the process, possibly mediated by the collagenase and hyaluronidase produced by group A streptococci. This liquefaction of fat results in separation of the skin from the underlying tissues, producing edematous fluid and the pathognomonic "dishwater pus," which has an offensive odor in the presence of anaerobes. If not swiftly managed surgically, infectious spread along the fascial planes of the neck can widely undermine the skin into the face and thorax. Further necrosis and liquefaction of the fat and fascia lead to arterial thrombosis, wet gangrene, and finally ischemic death of the skin. Exposed underlying muscle may be unaffected. Inflammatory factors lead to fever, tachycardia, and eventually, septic shock
10. In the presented case, although all of the subcutaneous fat and superficial fascia were necrotized, only a small portion of the skin was necrotized, though to be due to lack of blood supply. We also found that skin necrosis was affected by incision position. The incision is typically placed at the margin of the skin erythema area, where ample blood supply is difficult.
Clinicians must know that NF may accompany weakness, apathy, confusion, fever, tachycardia out of proportion to temperature elevation, hypotension, volume depletion, hypocalcemia due to calcium sequestration into areas of fat necrosis, and increased glucose level from increased gluconeogenesis from protein and hypoproteinemia
11. Green et al.
2 listed fever and signs and symptoms of systemic toxicity as diagnostic features of NF, including high fever, hypotension, prostration, and multiple organ failure. The condition is known as streptococcal toxin shock syndrome.
The clinically diagnosis of NF in the head and neck can be very difficult, especially in the initial stage, as the symptoms may resemble those of simple cellulitis or erysipelas. The distinguishing features are fast progression, pain, systemic toxicity, and presence of subdermal gas. This gas is best detected by CT scan
12. NF is diagnosed when the fascia is primarily infected. If muscle is primarily infected, then the diagnosis of streptococcal myositis or gas gangrene should be considered
13. Examination of a patient with NF will often show a lack of integrity of the subcutaneous fat, offering little resistance to the exploring finger. The skin will often feel undermined by the progressive infection
11.
When NF is suspected, prompt CT examination is mandatory. Among the other diagnostic radiography techniques, CT scan is the best way to detect the boundary and severity of NF. CT scan is more sensitive than other imaging modalities because it shows inflammatory changes, such as fascial edema and thickening or abscesses. In contrast to abscess, NF shows an area of fluid accumulation without significant rim enhancement at the periphery
14. Although gas accumulation is not a consistent finding, gas in the tissues had been considered a hallmark of NF. Another CT feature that may be considered characteristic of NF is a bizarre-shaped hypodense area, often irrespective of the cervical fascia compartment, that does not show significant rim enhancement on contrast injection
14. This feature is distinguishable from an abscess, which appears as a single or multilobulated area of fluid attenuation, usually confined to one compartment and typically showing peripheral rim enhancement. The bizarre shape of the hypodense area has been suggested to be caused by rapid spreading of the liquefaction necrosis across multiple layers of cervical fascia
15. In the presented case, a hypodense area without rim enhancement was observed, which is not characteristic of odontogenic abscess, and showed a small amount of pus, again in contrast to abscess.
Treatment involved securing the airway, broad-spectrum antimicrobial therapy, intensive care support, and prompt surgical debridement, repeated as needed
14. Airway management is critical with cervicofacial NF because the disease process may produce neck edema and necrosis, which increase the difficulty of intubation. Often with NF, extended airway management is warranted; in such situations, a tracheotomy is preferred over an endotracheal or nasotracheal tube
11. Gram staining may be helpful for selecting first-line antibiotics, and results of blood culture will most often be positive
10. Antibiotic therapy should be adjusted in response to culture results, but early triple-antibiotic therapy is the current standard of care
11. Reducing mortality rests on early diagnosis and prompt aggressive treatment. The early incision and debridement of all involved spaces can salvage the skin, which may still succumb to necrosis later in the disease course due to thrombolization of feeding vessels
12. The initial goals of surgical debridement are to eradicate all necrotic tissue and drain loculated collections and fluid in the fascial planes. Debridement with blunt dissection is undertaken until seemingly viable tissue is encountered. Following debridement, close monitoring for disease extension is critical. Because of the rapid progression of this disease, serial debridement is often performed under general anesthesia in the operating room within the first 24 hours after the initial debridement
2. Wound and blood cultures should be performed to direct proper antibiotic therapy. The wounds should be left open and loosely packed with gauze. This dressing should be changed multiple times per day. In addition, wounds should be irrigated each time the dressing is changed, and one should be observant for the redevelopment of necrosis
16. General agreement is that the defects should be kept open, and debridement should be repeated until completely healthy granulating tissue is obtained
17. On average, an NF patient undergoes three surgeries
11. Appropriate nutritional support, pain management, fluid resuscitation, and replacement of electrolytes are important measures for patient survival
11. Unfortunately, septic shock can develop, which will lead to multiple organ failure and death
18.
Major complications from NF are mediastinal involvement, septic shock, pleural effusion, lung empyema, airway obstruction, rupture of major vessels, brain abscess, disseminated intravascular coagulopathy, sepsis, acute renal failure, and respiratory failure. Usually, such complications in immunocompromised patients, such as diabtes mellitus and cancer patients, lead to death
7,
14. Most deaths occur in patients in whom the infection is complicated by mediastinitis. Banerjee et al.
19 found that 44% of the cervical NF cases in their series had mediastinal involvement, 38% of whom died of the disease. Infection can propagate from the head and neck to the mediastinum through the retropharyngeal or prevertebral space or can descend along the carotid sheath. Direct downward extension of fasciitis involving the pretracheal fascia can also result in mediastinal or pleural involvement
20.
Immediate reconstruction is not recommended after recovery from the infection. Such procedures can be considered once a stable wound edge is observed. Primary closure can be performed with various local flaps, skin graft, regional flaps and even free vascularized flaps
16. In the presented case, the wound margin was necrotized; after ample delay, the wound was reconstructed using a local flap.
Go to :


