Abstract
Angiography is the gold standard for the diagnosis and complete resection of arteriovenous malformations (AVMs). The absence of residual AVM after surgery is commonly believed to reduce the risk of future hemorrhage. However, AVMs can recur after proven complete angiographic resection can occur, albeit rarely, especially in the pediatric population. We report a rare case of a recurrent AVM two years after complete resection in an adult patient. This case report shows that AVMs in adults can recur despite their rarity and despite postoperative angiography confirming complete removal. Moreover, in this case, the recurrent AVM involved a new feeding vessel that was not involved with the initial lesion.
The term "vascular malformation" is a generalized term used to describe a group of lesions formed by abnormal angiovascular or lymphovascular structures. Arteriovenous malformations (AVMs), particularly in the head and neck, are high-flow vascular anomalies. These lesions have direct communication between an artery and vein, bypassing the capillary bed12, and AVMs have a gradual onset and progression. In the oral cavity, AVMs can present at any site, but they occur most commonly on the buccal and gingival mucosa and on the anterior two-thirds of the tongue and palate3.
These lesions can be diagnosed by plain radiography, computed tomography (CT), magnetic resonance imaging (MRI), or angiography. A sclerosing agent and embolization combined with surgical treatment is still the most modern approach to treat these lesions4. Complete surgical excision of AVMs with confirmatory postoperative angiography is considered a cure. However, recent reports suggest that AVMs in children may recur after negative postoperative angiograms, which may reflect the immaturity of their vasculature5. This report describes a recurrent AVM on the palate of a 38-year-old male patient after initial embolization and surgical resection.
This study followed the medical protocol and esthetics of the Declaration of Helsinki. Additionally, the regional ethical review board of the Pusan National University Dental Hospital Institutional Review Board (IRB No. PNUDH-2015-010) approved this study.
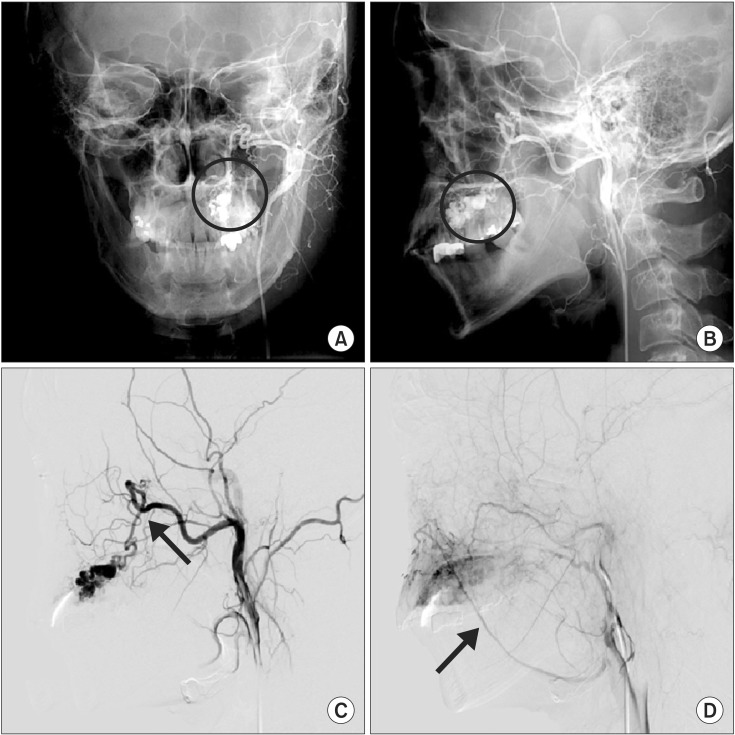
A previously healthy 38-year-old male presented to our hospital in September 2010 with palatal swelling and spontaneous hemorrhage. Clinical examination, including inspection and palpation, revealed fluctuant palatal swelling and ulceration. On dental panorama and CT, there was no evidence of definite palatal cortical bone resorption.(Fig. 1) MRI showed an enhancing lesion of the left palate with abnormal vascular signals.(Fig. 2) To further evaluate, the patient underwent angiography and was ultimately diagnosed with an AVM of the left palate. This AVM was supplied by the descending palatine artery and was drained through a facial vein.(Fig. 3)
On November 22, 2010, the patient underwent embolization using an Onyx (Covidien/Ev3 Neurovascular, Irvine, CA, USA) injection in the descending palatine artery via a unilateral femoral artery approach under general anesthesia. Obliteration of the palatal AVM was confirmed by postprocedure angiography.(Fig. 4) After embolization therapy, the AVM was successfully resected under general anesthesia. The postoperative biopsy results confirmed an AVM. A palatal stent was inserted after the resection, and the patient made an uneventful recovery. Although MR angiography (MRA) was recommended at the eight-month follow-up visit, the patient was lost to follow-up.
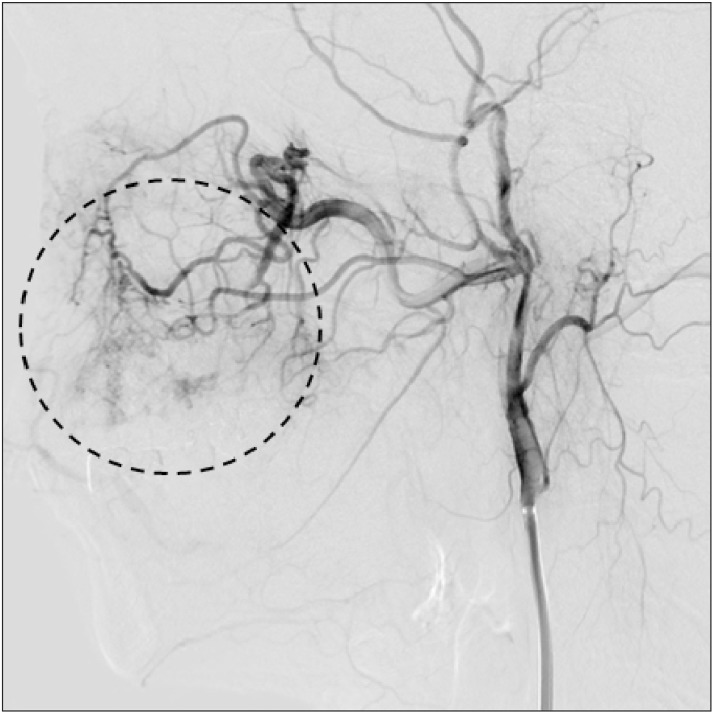
Thirty-one months after surgery, at the age of 41 years, the patient developed a pulsatile mass on his palate accompanied by ulceration and swelling. CT angiography (CTA) revealed recurrence of his AVM on the left palate.(Fig. 5) The recurrent lesion was larger than the former lesion, and the recurrent AVM was supplied simultaneously by the descending palatine artery and the posterior superior alveolar artery. The facial vein supplied drainage, as with the initial AVM. The patient underwent an uneventful second Onyx embolization, and obliteration of the palatal AVM was again confirmed by angiography.
The early vascular anomaly classification system introduced by Virchow6 characterized vascular lesions according to the pathologic appearance of the vessel. In 1982, Mulliken and Glowacki resolved this confusion through a classification system of vascular birthmarks7. They grouped birthmarks into two major categories: hemangiomas and malformations. In 1996, this classification system was modified slightly to reflect other types of vascular tumors that exhibit different clinical and histological characteristics8. Consequently, the updated International Society for the Study of Vascular Anomalies (ISSVA) classification system now divides vascular birthmarks into vascular tumors and vascular malformations.
AVMs are high-flow lesions with direct communication between an artery and vein, bypassing the capillary bed12. Head and neck AVMs, including palatal AVMs, are rare, and their origin and pathogenesis are unclear. However, it is known that trauma, ischemic events secondary to thrombosis, hormonal changes, puberty, and ectasia can induce proliferation of AVMs and trigger lesions development and troublesome symptoms9.
In the oral cavity, AVMs can present at any site but occur most commonly on the anterior two-thirds of the tongue, palate, and buccal and gingival mucosa3. Intraosseous AVMs in alveolar bone often present with tooth mobility, pericoronal bleeding, and sometimes occlusal anomalies10. In the present case, the palatal lesions were associated with spontaneous bleeding and ulceration. AVMs are by far the most difficult vascular anomaly to manage due to replacement of the normal tissue with diseased vessels and also due to the very high rate of recurrence.
When diagnosing AVMs, plain radiography, CT, MRI, or angiography can be used. In the maxilla and mandible, the AVMs produce a poorly defined radiolucent image, often having the appearance of soap bubbles or honeycombing, with small, irregular, and rounded lacunae. MRI has become the imaging modality of choice because the extent of the lesions and invasion status can be characterized. In addition, angiography is useful in poorly defined cases and for embolization therapy prior to surgery11. This modality demonstrates flow characteristics and feeding vessel anatomy and identifies dangerous anastomoses.
Many treatment options are available for AVMs, including endovascular or percutaneous embolization, sclerotherapy, and surgical excision4. However, surgical excision is often difficult and carries the potential risk of hemorrhage and damage to the surrounding structures. Accordingly, transcatheter embolization plays a significant role in the treatment of patients with AVMs12. Preoperative embolization prior to surgical intervention may help reduce the risk of massive bleeding. Therefore, a combination of preoperative embolization and surgical resection is commonly used for head and neck AVMs.
Angiography is the gold standard for both diagnosing AVMs and identifying their complete resection after surgery 11. The absence of residual AVM after surgery is thought to be reassuring for avoiding future hemorrhage. However, the recurrence of AVMs following an angiographically proven complete resection can occur, particularly in the pediatric population. This paper reports a rare case of AVM recurrence after embolization followed by surgical resection in a 38-year-old male patient. Weil et al.13 reported that this occurrence is rare, with only 29 cases being reported in the English literature. In a surgical series of AVM treatment, AVM recurrence has been reported in 0.6% to 13% of cases14, but the actual rate of recurrence is unknown. Recurrence rates may be affected by selection bias in studies that include cases of recurrent hemorrhage without proven AVM recurrence or in studies excluding undetected, asymptomatic recurrences due to lack of systematic follow-up angiography across all institutions.
AVMs are arteriographically classified into three types of "extratruncular" lesions based on the morphology of their nidus: 1) arterio-venous fistulae, 2) arteriolo-venous fistulae, and 3) arteriolo-venulous fistulae. Complete obliteration of the AVM nidus through embolization therapy offers a better prognosis; however, in some cases, such as type III AVMs, complete injection of embolization materials into the nidus can be difficult15. In the present study, our patient had a type III AVM with a few nidi remaining in the palate after embolization. Thus, recurrence of this palatal AVM after complete occlusion of the feeding artery might have been due to these residual nidi, in addition to the pathology of the vessel itself. The posterior superior alveolar artery did not initially act as a feeding artery for this AVM, as was shown via initial angiographic imaging. Considering these findings, especially the secondary embolization imaging, we assumed that this recurrent AVM was due to new pathology, such as capillary malformation combined with an AVM, rather than a residual, unembolized posterior superior alveolar artery.
With AVM recurrence following complete removal, the timing of follow-up angiography is brought into question. The consensus is that digital subtraction angiography (DSA) should be performed immediately after surgery to rule out any residual AVM; however, this approach is known to increase the risk of immediate hemorrhage, warranting early re-operation16. However, AVM recurrence after complete resection has been hypothesized to portend an increased risk of hemorrhage, which might have catastrophic consequences 17. Therefore, investigation for an asymptomatic, recurrent AVM might be warranted because subsequent treatment can reduce the hemorrhage risk, preventing morbidity and mortality. In adults, follow-up angiography long after treatment is not often recommended because AVM recurrence is rare and angiography is not without risk. For children, most authors recommend a second DSA at 6 or 12 months in all AVM cases with initial negative angiography18. MRA and CTA are less than DSA invasive alternatives and have been used to detect recurrent AVMs after an initial complete resection. Their sensitivity, however, is lower than that of DSA, and they should probably not be used in place of the standard early postoperative imaging after complete surgical removal.
In conclusion, complete surgical excision documented by postoperative angiography is considered an AVM cure and is believed to eliminate the risk of subsequent hemorrhage. This case report shows that AVMs in adult patients can recur despite complete removal confirmed by postoperative angiography. Moreover, in this case, the recurrent AVM had a new feeding vessel not involved with the initial lesion. This case suggests that postoperative follow-up angiography should be considered, even if the AVM was completely resected.
Acknowledgements
Authors' contributions: YHS collected the data and wrote the manuscript. SKB gave important input and carefully reviewed the manuscript. MSK assisted to collect and organize radiographic data. YDK contributed significantly to the treatment of the patient and designed this study and interpreted the data. All authors read and approved the final manuscript.
References
1. Chiu YW, Wu HT, Chen YW, Lui MT, Kao SY, Lo WL. A giant venous malformation of face and neck: a case report. Taiwan J Oral Maxillofac Surg. 2011; 22:110–117.
2. Duncan IC, Fourie PA. Vascular malformations part 2: current classification of vascular malformations. South Afr J Radiol. 2004; 8:23–30.
3. Shetty DC, Urs AB, Rai HC, Ahuja N, Manchanda A. Case series on vascular malformation and their review with regard to terminology and categorization. Contemp Clin Dent. 2010; 1:259–262. PMID: 22114434.
4. Noreau G, Landry PP, Morais D. Arteriovenous malformation of the mandible: review of literature and case history. J Can Dent Assoc. 2001; 67:646–651. PMID: 11841745.
5. Sonstein WJ, Kader A, Michelsen WJ, Llena JF, Hirano A, Casper D. Expression of vascular endothelial growth factor in pediatric and adult cerebral arteriovenous malformations: an immunocytochemical study. J Neurosurg. 1996; 85:838–845. PMID: 8893722.

6. Virchow R. Die krankhaften Geschwülste 1. Berlin: Hirschwald;1863.
7. Choi DJ, Alomari AI, Chaudry G, Orbach DB. Neurointerventional management of low-flow vascular malformations of the head and neck. Neuroimaging Clin N Am. 2009; 19:199–218. PMID: 19442906.

8. Garzon MC, Huang JT, Enjolras O, Frieden IJ. Vascular malformations: part I. J Am Acad Dermatol. 2007; 56:353–370. PMID: 17317485.
9. Richter GT, Friedman AB. Hemangiomas and vascular malformations: current theory and management. Int J Pediatr. 2012; 2012:645678. DOI: 10.1155/2012/645678. PMID: 22611412.

10. Anderson JH, Grisius RJ, McKean TW. Arteriovenous malformation of the mandible. Oral Surg Oral Med Oral Pathol. 1981; 52:118–125. PMID: 6943478.

11. Codd PJ, Mitha AP, Ogilvy CS. A recurrent cerebral arteriovenous malformation in an adult. J Neurosurg. 2008; 109:486–491. PMID: 18759581.

12. Jacobowitz GR, Rosen RJ, Rockman CB, Nalbandian M, Hofstee DJ, Fioole B, et al. Transcatheter embolization of complex pelvic vascular malformations: results and long-term follow-up. J Vasc Surg. 2001; 33:51–55. PMID: 11137923.

13. Weil AG, Li S, Zhao JZ. Recurrence of a cerebral arteriovenous malformation following complete surgical resection: a case report and review of the literature. Surg Neurol Int. 2011; 2:175. PMID: 22276230.

14. Klimo P Jr, Rao G, Brockmeyer D. Pediatric arteriovenous malformations: a 15-year experience with an emphasis on residual and recurrent lesions. Childs Nerv Syst. 2007; 23:31–37. PMID: 17053936.

15. Lee BB, Baumgartner I, Berlien HP, Bianchini G, Burrows P, Do YS, et al. International Union of Angiology. Consensus Document of the International Union of Angiology (IUA)-2013. Current concept on the management of arterio-venous management. Int Angiol. 2013; 32:9–36. PMID: 23435389.
16. Spetzler RF, Hargraves RW, McCormick PW, Zabramski JM, Flom RA, Zimmerman RS. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992; 76:918–923. PMID: 1588424.

17. Gabriel EM, Sampson JH, Wilkins RH. Recurrence of a cerebral arteriovenous malformation after surgical excision: case report. J Neurosurg. 1996; 84:879–882. PMID: 8622165.
18. Andaluz N, Myseros JS, Sathi S, Crone KR, Tew JM Jr. Recurrence of cerebral arteriovenous malformations in children: report of two cases and review of the literature. Surg Neurol. 2004; 62:324–330. PMID: 15451278.

Figure. 1
A. Preoperative photograph shows left palatal swelling and ulceration. B, C. Dental panorama and computed tomography reveal no definite cortical bone resorption or specific lesion.
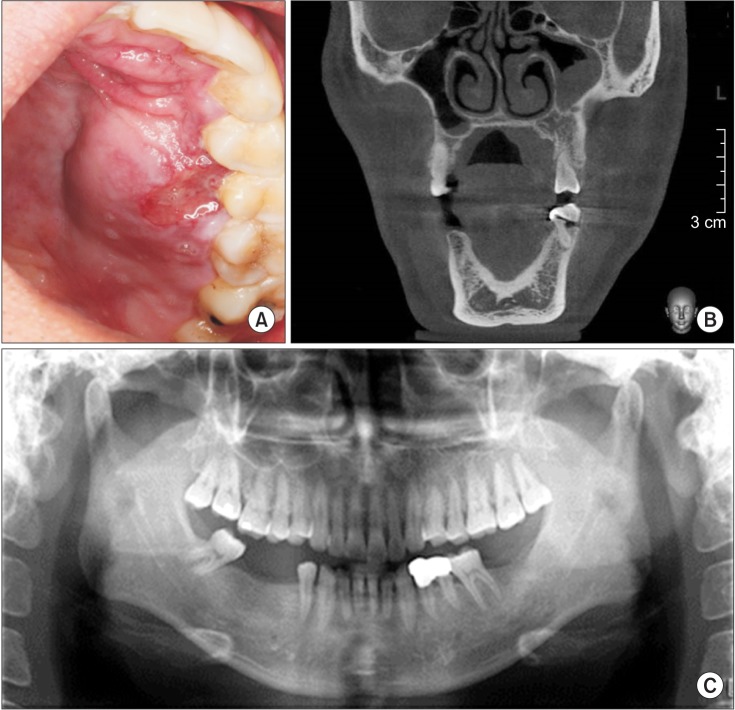
Figure. 2
Preoperative axial T1-weighted (A) and T2-weighted (B) magnetic resonance images show a high-intensity mass on the left palate. Coronal T1-weighted images (C, D) reveal that the lesion did not involve the maxilla.
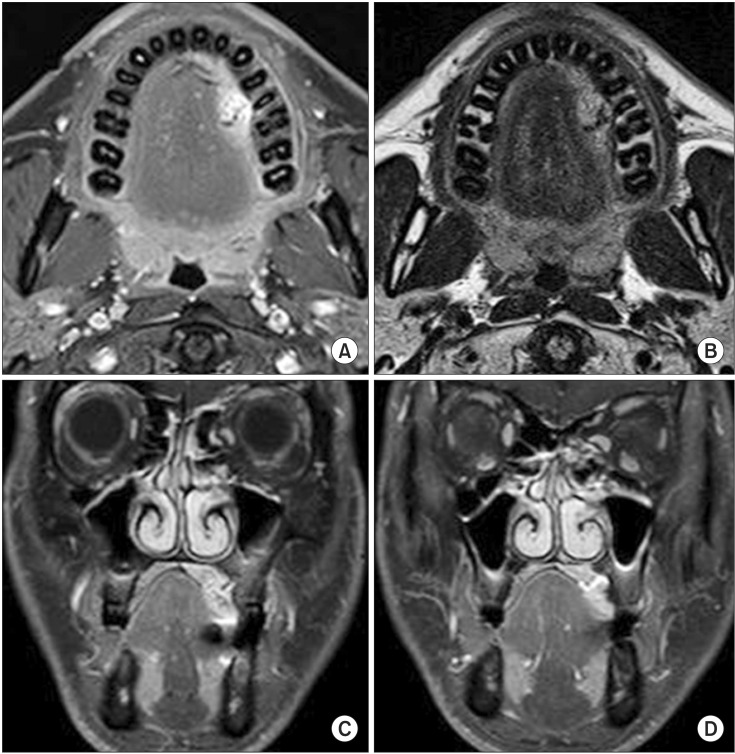
Figure. 3
A, B. Preoperative left carotid angiogram show an arteriovenous malformation in the left palate (circles). This lesion is supplied by the descending palatine artery (C, arrow) and drained through a facial vein (D, arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download