Abstract
Objectives
This study was performed to evaluate the impact of glycosylated hemoglobin (HbA1c) level on characteristics and prognosis of maxillofacial fascial infection in diabetic patients.
Materials and Methods
We reviewed the medical records of 72 patients (35 patients with HbA1c lower than 7.0% and 37 patients with HbA1c higher than 7.0%) diagnosed with maxillofacial fascial space infection and hospitalized for treatment at the Department of Oral and Maxillofacial Surgery in Dankook University Hospital (Cheonan, Korea) from January 2005 to February 2014. We compared demographics, parameters of glucoregulation (HbA1c), laboratory parameters of inflammation (white blood cell [WBC], C-reactive protein [CRP] count), type and number of involved spaces, type and number of antibiotics, period of hospitalization, number of surgical operations, need for tracheostomy, complications, computed tomography (CT), and microorganisms between the two groups.
Results
Compared with the well-controlled diabetes mellitus (DM) group (HbA1c <7.0%), patients in the poorly-controlled (HbA1c ≥7.0%) DM group had the following characteristics: longer hospitalization periods, higher values of laboratory parameters of inflammation (WBC, CRP count) at the time of admission, higher number of antibiotics prescribed, more frequent complications, frequent deep neck space involvement, and distinctive main causative microorganisms. As the HbA1c level increases, hospitalization periods and incidence of complications increase gradually.
Risk of infection depends on several factors, including host defense mechanisms, functional or anatomical abnormalities of the host, and virulence of the infecting microorganism. It is not only the host defense that determines the outcome of infection, but the timing and appropriateness of antimicrobial treatment as well. There are two main routes of infection in oral and maxillofacial infections; one is the route from the root apex and the other is the route from the deep periodontal pocket. Infection sometimes expands into the fascial space directly and causes a fascial space infection. The fascial space is a potential space that can be expanded by purulent exudate. The fascial spaces that may be directly affected by odontogenic infections are called 'primary spaces', such as the canine, buccal, infratemporal, submental, sublingual, or submandibular spaces. Failure to control primary spaces infection may cause them to spread to 'secondary spaces', such as the submasseteric, pterygomandibular, and temporal spaces. Infections that are not treated properly can spread beyond secondary spaces to deep neck fascial spaces such as the lateral pharyngeal, retropharyngeal, and prevertebral spaces. It is difficult to treat patients who have these space infections without drainage of the purulent exudates, since those areas are surrounded by connective tissues that have poor blood supply1.
The term "diabetes mellitus (DM)" describes a metabolic disorder of multiple etiology characterized by chronic hyperglycemia with disturbances of carbohydrate, fat, and protein metabolism, resulting from defects in insulin secretion, insulin action, or both. Diabetes is usually diagnosed based on plasma glucose criteria, either by fasting plasma glucose (FPG) or the 2-hour plasma glucose (2-h PG) value after a 75-g oral glucose tolerance test (OGTT). Recently, an International Expert Committee added glycosylated hemoglobin (HbA1c) as a third option to diagnose diabetes23. Patients with DM have higher risks of infection due to abnormal phagocytosis, persistent reduction of blood flow, and cell-mediated immune abnormalities typical of diabetic patients456.
HbA1c is a form of hemoglobin that is measured primarily to identify the average plasma glucose concentration over the previous two to three months prior to the measurement. Because HbA1c testing can be performed at any time of day and without special patient preparation, it is more convenient for patients and health care providers compared to the oral glucose tolerance test or measuring FPG7.
There are many studies comparing diabetics and nondiabetics with infections in the maxillofacial region via blood glucose level at the time of admission or with a past medical history of DM89101112. However, admission glucose levels or fasting glucose levels are subject to changes of daily activities, such as diet content, amount of exercise, and physical or emotional stress before examination, even in well-controlled DM before admission13. On the other hand, HbA1c reflects long-term glycemic status and is a more stable measurement than FPG7. Therefore, HbA1c might be a better indicator for glucose control in diabetes than fasting blood sugar14. However, scarce studies exist on infection of DM patients with different HbA1c levels in the oral and maxillofacial surgery fields. Therefore, this study was conducted to evaluate the clinical impact of HbA1c level and regulation of DM on the characteristics and prognosis of oral and maxillofacial fascial infection in patients with DM.
We reviewed the medical records of patients diagnosed with maxillofacial fascial space infection and hospitalized for treatment at the Department of Oral and Maxillofacial Surgery in Dankook University Hospital (Cheonan, Korea) from January 2005 to February 2014. Among these patients, we selected patients with DM from records of previous medical history of DM or patients who newly diagnosed with DM during admission after consultation with the Department of Endocrinology. Patients diagnosed with secondary infections from fractures, osteomyelitis, or cyst formation, patients hospitalized only for dentoalveolar abscess, and patients who were administered antibiotics before admission were excluded from this study. A total of 72 patients were included in this study. The HbA1c levels of all patients at the time of admission were evaluated. We divided these patients into two groups according to HbA1c level.
· Group 1: Thirty-five patients with DM with HbA1c level under 7.0% (HbA1c <7.0%) and included 18 male and 17 female patients.
· Group 2: Thirty-seven patients with DM with HbA1c level over 7.0% (HbA1c ≥7.0%) and included 20 male and 17 female patients.
Investigations between the two groups regarding patient age, parameters of glucoregulation (HbA1c), laboratory parameters of inflammation (white blood cell [WBC], C-reactive protein [CRP] count), type and number of involved spaces, type and number of antibiotics, period of hospitalization, number of surgical operations, need for tracheostomy, complications, computed tomography (CT), and type of microorganism were performed.
Laboratory values included indicators such as WBC count on admission and at discharge and CRP at admission and at discharge to evaluate severity and subsidence of infection.
Three-dimensional neck CT with enhancement was used to identify which fascial space was involved with infection for all patients. The same examiner read all CT images and diagnosed the infection.
Immediately after admission, we administered intravenous antibiotics and fluid therapy for all patients and obtained a CT image as soon as possible in accordance with nil per os time. Amoxicillin/clavulanic acid (augmentin) was used as the first drug of choice. Clindamycin and a cephalosporin were subsequently used empirically according to the progress of laboratory tests. We performed emergency operations for patients complaining of dysphagia, dyspnea, or reduced oxygen saturation immediately after obtaining CT imaging. In cases of non-emergency patients, we performed elective operations after localization of the abscess. We performed incision and drainage with insertion of silastic drain under general anesthesia or local anesthesia in accordance with the status of the patient. Tracheostomy was performed on any patient with questionable postoperative airway patency. Pus samples from the site of infection were collected during incision and drainage (I&D), using sterile agar gel transport swabs. Sixteen patients among group 1 and 23 patients among group 2 yielded identifiable pathogens. Susceptibility to various antibiotics was evaluated and antibiotics were tailored to culture results.
We performed daily dressing changes for the operation site and treatment was terminated when clinical and radiographic signs improved and after CRP and WBC were normalized.
For statistical analysis between the two study groups, we performed a t-test, Mann-Whitney test, logistic regression test, chi-square test, and linear regression analysis (IBM SPSS Statistics version 21.0; IBM Co., Armonk, NY, USA). Data were presented as the mean±standard deviation (SD). A P-value less than 0.05 was considered to be significant.
The average patient age in group 1 and group 2 was 60.9 years (range, 32-88 years) and 63.2 years (range, 41-85 years), respectively. The age showed no statistically significant difference between the two groups (P>0.05). The average level of HbA1c level was 6.0% (range, 4.6%-6.9%) in group 1 and 9.5% (range, 7.0%-14.3%) in group 2.(Table 1)
The mean hospitalization days of group 1 and group 2 was 9.6 days (range, 4-36 days) and 15.1 days (range, 4-66 days), respectively. Patients in group 2 had longer hospitalizations than those in group 1.(Table 2) This showed a statistically significant difference between the two groups. In the linear regression analysis, hospitalization days increased by 2.436 days as HbA1c levels increased by 1%.(Table 3)
WBC count and CRP of both groups was increased at the time of admission.(Table 4) This pre-operative WBC count and CRP was higher in group 2 than in group 1 and showed statistical significance (P<0.05). At the time of discharge, these two laboratory parameters showed almost normal values and there were no statistically significant differences between the two groups in terms of these two parameters (P>0.05).
The submandibular, buccal, and submental spaces were the most commonly involved spaces.(Table 5) The range of the number of involved spaces was one through five in group 1 and one through nine in group 2. The mean±SD number of involved spaces was 1.62±1.0 and 2.16±1.7 in group 1 and group 2, respectively. However, this did not show statistically significant differences between the two groups (P=0.10). There were 14 patients (40.0%) in group 1 and 21 patients (56.8%) in group 2 with multiple-space infection (more than 2 spaces). Note that deep neck space infections such as the retropharyngeal or prevertebral space were seen only in group 2.
The tracheostomy ratio was 5.7% (2 patients) and 13.5% (5 patients) in groups 1 and 2, respectively (P>0.05). Patients in group 2 needed a higher number of antibiotics for treatment. The mean number of prescribed antibiotics was 2.0 (range, 2-3) in group 1 and 2.4 (range, 1-6) in group 2, which was a statistically significant difference (P=0.039). However, the mean number of surgical procedures was not significantly different between the two groups (P=0.56).
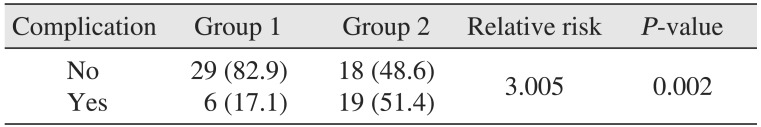
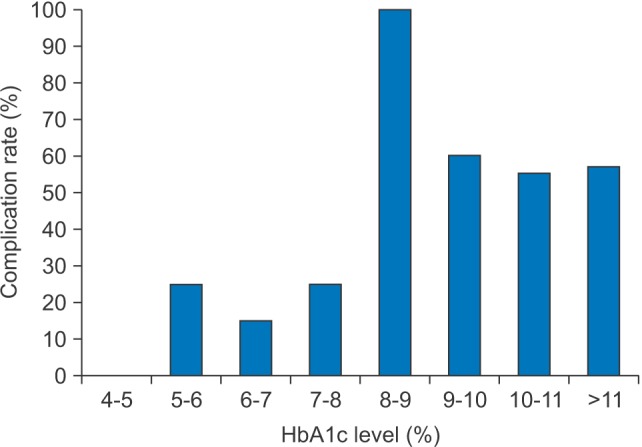
The ratio of patients with complications was 17.1% (6 patients) in group 1 and 51.4% (19 patients) in group 2. The most commonly encountered complications were airway obstruction and skin defects such as necrotic skin, pitting edema, and fistula formation. There was one patient in group 2 that expired due to septic shock.(Table 6) Chi-square analysis on the prevalence of complications between the groups revealed a significantly higher prevalence in group 2 compared to the group 1 (relative risk=3.005, P=0.002).(Table 7) Logistic regression analysis showed that as HbA1c levels increase by 1, the prevalence of complication increases by a factor of 1.404, which was found to be significant.(Table 8) Patients were categorized by 1% of HbA1c level and linear by linear analysis was performed.(Fig. 1) The prevalence of complications was low, even with an HbA1c level lower than 7% to 8%; however, complications increased drastically in groups with an HbA1c level greater than 8% to 9%.
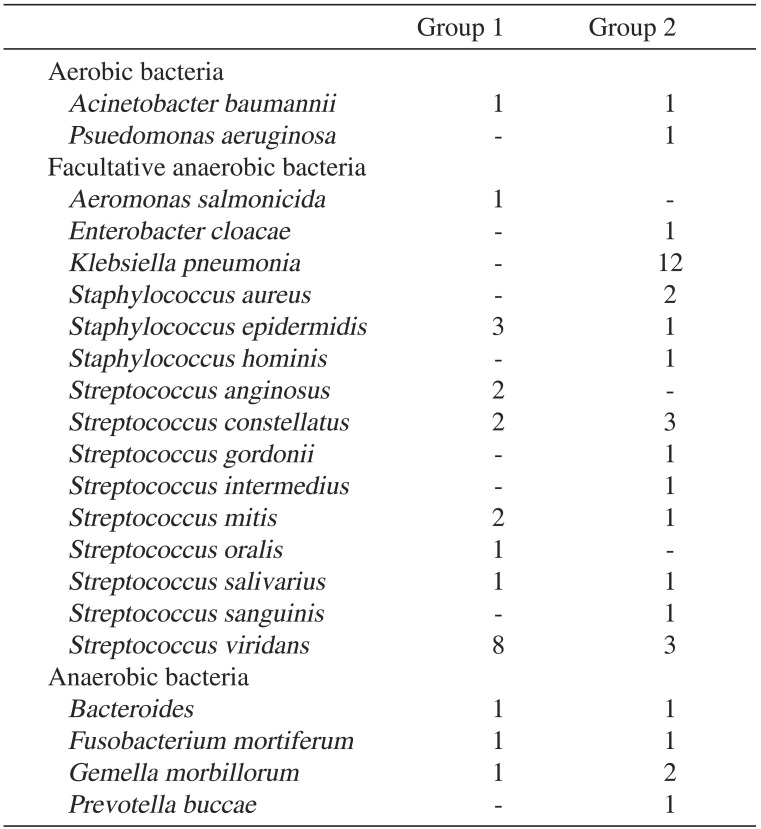
In total, there were 16 patients in group 1 and 23 patients in group 2 that yielded identifiable pathogens.(Table 9) In group 1, the most common microorganism cultured was Streptococcus viridans (33.3%, 8/24), followed by Staphylococcus epidermidis (12.5%, 3/24). In group 2, the most common microorganism cultured was Klebsiella pneumonia (34.3%, 12/35) followed by S. viridans (8.6%, 3/35) and Streptococcus constellatus (8.6%, 3/35).
DM is not only a predisposing factor for common infections, but also responds poorly to infections once they have developed, especially when glucose levels are uncontrolled81516. The mechanisms in which diabetes predisposes to infection may be attributable to hyperglycemia. Many factors increase susceptibility of hyperglycemia to infections. Hyperglycemia has adverse effects on the immune system, causing impaired chemotaxis, adherence of microorganisms to polymorphonuclear leukocytes and lymphocytes, and disruption of phagocytosis. Hyperglycemia reduces the ability of WBCs to break down phagocited microorganisms. Since the process of phagocytosis is a basic defense against bacteria and fungi, the disruption of this process is thought to be responsible for a higher incidence of infections in diabetics17. Geerlings and Hoepelman18 suggested that the function of neutrophils, such as chemotaxis or production of cytokines, is reduced under high blood sugar levels. These defects of the immune system along with vascular abnormalities render diabetic patients at higher risk for a variety of invasive infections19. Chronic diseases such as diabetes occur more often in older patients. In this study, the average age of the well-controlled DM group and the poorly-controlled DM group was 60.9 years and 63.2 years, respectively.
There have been many studies comparing diabetics and nondiabetics with infections in the maxillofacial region. Many of them divided the groups by blood glucose level on admission or past medical history of DM. They concluded that diabetic patients are more likely to develop complications, greater incidence rates of involved spaces, and abnormal hematologic findings192021. Sim et al.13 reported that physical and emotional stress increases blood glucose level via activation of both the adrenergic and glucocorticoid systems. Koraćević et al.22 reported that increased glucose levels during the stress might be a result of sympathetic nervous system activation, which raises the production of catecholamines that stimulate processes of glyconeogenesis, glycogenolysis, and liposysis. In addition, infection itself can be a cause of hyperglycemia. One of the most important metabolic features of an infection is catecholaminemia, and it may disrupt the regulation of blood glucose in four ways: a) increased gluconeogenesis, b) reduction of the intrinsic secretion of insulin, c) increased resistance to intrinsic insulin, and d) increased utilization of glucagon23. Therefore, blood glucose levels on admission may change at the time of admission even if glucose metabolism was previously well controlled before admission in diabetic patients.
On the other hand, the HbA1c level appears to be a more reliable marker for average glycaemia in the last two to three months and seems to be a better measurement for the evaluation of glucose regulation on infection.
The American Diabetes Association recommends that HbA1c should be below 7.0% for glycemic targets for most patients with diabetes. Lowering HbA1c below or around 7.0% has been shown to reduce microvascular complications and macrovascular disease. Therefore, we set the borderline level of HbA1c between the two groups at 7.0%. However, this standard only considers complications of DM itself, and lacks concerns regarding prognosis after surgical procedures or certain other diseases. There are some reports on diseases and postoperative complications according to the HbA1c levels in diabetic patients. Jupiter et al.24 reported a significant difference of prevalence of postoperative complications after ankle and foot surgery between well-controlled diabetic patients (HbA1c <7.0%) and poorly-controlled diabetic patients (HbA1c >7.0%)8. Halkos et al.25 reported that after coronary artery bypass surgery of diabetic patients, postoperative complications such as infection, myocardial infarction, and arterial fibrillation occurred significantly more in patients with HbA1c levels higher than 7.0%. Dronge et al.26 reported that after performing urologic, vascular, or orthopedic surgery in diabetic patients, the prevalence of postoperative infection was higher in patients with higher HbA1c levels. However, studies on the prognosis of fascial infection in diabetic patients according to the HbA1c levels in maxillofacial surgery field are rare.
Lee et al.27 reported that 81.6% of odontogenic infection showed single fascial space involvement. In contrast, Rega et al.28 reported that multiple-space infections were more common than single-space infections in odontogenic infection. In our study, 34 patients (48.6%) had more than two fascial space involvements in both groups. The most commonly involved fascial space in this study was the submandibular space followed by the buccal space in both groups. This result was similar to other previously reported studies1921. Deep neck infections, such as lateral, retropharyngeal, or prevertebral spaces may result in life-threatening complications including upper airway obstruction, descending mediastinitis, jugular vein thrombosis, venous septic emboli, carotid artery rupture, septic shock, and disseminated intravascular coagulopathy2930. Thus, these infections require early diagnosis and aggressive treatment. Note that the retropharyngeal (3/80) and prevertebral (2/80) spaces were involved only in the poorly-controlled DM group. This result of our study is similar to that of a previous study of Huang et al.10, who reported that hyperclycemic patients are vulnerable to deep neck infections.
There are many studies reported that periods of hospitalization were longer in DM patients, and WBC and CRP levels were higher in DM patients compared to non-DM patients202131. Similarly, in this study, patients in the poorlycontrolled DM group (group 1) had a comparatively longer hospital stay compared to the well-controlled DM group (group 2) (P<0.05). Regardless of the group, HbA1c level and hospitalization period were in a linear relationship, with the latter increasing as the former increases. As the HbA1c level increased by 1%, hospitalization period increased by 2.4 days.(Table 3) WBC count and CRP levels at time of admission were significantly higher in the poorly-controlled DM group compared to the well-controlled DM group (P<0.05). These results can be said to be a reflection of persistent inflammation and delayed healing, which could be due to a prolonged inflammatory response to cytokine dysregulation and enhanced fibroblast apoptosis by hyperglycemia3233.
Amoxicillin/clavulanic acid (augmentin) was used first as the drug of choice. Clindamycin and cephalosporin were subsequently used empirically according to the progress of laboratory tests. In patients who underwent a pus culture test, we tailored antibiotics according to the sensitivity results. The mean number of antibiotics prescribed was higher in the poorly-controlled DM group compared to the well-controlled DM group. Number of I&D or the tracheostomy ratio were not significantly higher in the poorly-controlled group. The poorly-controlled DM group had more frequent and severe complications. In addition, the prevalence of complications increased consistently as HbA1c levels increased; at a 1% increase of HbA1c level, the prevalence of complications increased by a factor of 1.4.(Table 8) The major complication was airway obstruction followed by skin defects such as skin necrosis, fistula formation, exfoliative dermatitis, and pitting edema. Skin defects might be, to a larger extent, related to the operation site and delayed healing, due to poorly-controlled glucose levels. Severe complications such as sepsis and descending mediastinitis were seen in the poorly controlled DM group only and one patient in this group died due to septic shock and pneumonia. These results are consistent with the findings of previous studies122034. As seen in Fig. 1, the prevalence of complications increased by a small extent until the 7% to 8% range, but increased drastically for the 8% to 9% range and continued. Therefore, an HbA1c level of 8% could be considered as a reference point for predicting the prevalence of complications in maxillofacial infection patients.
The bacteriologic patterns of maxillofacial infection are usually polymicrobial, including aerobes, microaerophilics, and anaerobes. Many studies reported that K. pneumonia and Streptococcus spp. were the most commonly isolated organisms among diabetic patients. Streptococcus spp. and Staphylococcus spp. are the most commonly isolated organisms in non-diabetic patients110122728. In our study, Streptococcus spp. was most commonly isolated in group 1. This causative microorganisms of the well-controlled DM group were similar to those of the non-diabetic patients. On the other hand, in group 2, K. pneumonia was the most commonly isolated. This K. pneumonia plays a role in deep neck infection10. Possible contributing factors of the preponderance of K. pneumonia include increased oropharyngeal colonization by gram-negative bacilli, and defects of host defenses, especially phagocytic function impairment in hyperglcaemia35. Clindamycin and other antibiotics are often used to treat head and neck infections, but should not be administered alone for the therapy of infection in poorly controlled DM patients because of their limitation in controlling K. pneumoniea. Thus, clinicians should take into account the preponderance of this microorganism when choosing empirical antibiotics.
Phagocytic function has been shown to be compromised when glucose levels range from 198.6 to 270.8 mg/dL (11 to 15 mmol/L). Therefore, glucose control plays an important role in the treatment strategy of hyperglycemic patients with infection15. There is a report that patients with high HbA1c levels tend to have more acute infections, not only in maxillofacial regions but in general as well23. Moreover, in a study of influence of glycemic control on outcomes of common infections, Leibovici et al.36 reported that in patients without a fatal underlying disease, a higher fatality rate could be demonstrated in patients with higher HbA1c levels. Diabetic patients with poor glycaemic control were at high risk for a fatal outcome. It has been postulated that a poorly controlled diabetic state predisposes the patient to sepsis, and once this disease is established further, adverse carbohydrate metabolism may result. A recent report on diabetes control and infections showed that impaired neutrophil bactericidal function is associated with poor blood glucose control and that it is likely that neutrophil bactericidal function will improve as blood glucose control improves37. In addition to the aggressive treatment that is needed in diabetic patients compared to non-diabetic patients when infection occurs, we also found that regulation of blood glucose (HbA1c within 7.0%) is critical to the prognosis of infection. HbA1c levels of 7.0%, which are suggested by the ADA, were confirmed to be a reliable marker for prognosis in oral and maxillofacial surgery patients with infections.
In conclusion, this study confirmed that good preoperative glycemic control, as measured by HbA1c levels less than 7%, is associated with a significantly better prognosis in oral and maxillofacial fascial infection. This simple laboratory test drawn preoperatively may provide the clinician with a more accurate risk profile and provide additional prognostic information when discussing morbidity with patients and their families.
References
1. Chang CM, Lu FH, Guo HR, Ko WC. Klebsiella pneumoniae fascial space infections of the head and neck in Taiwan: emphasis on diabetic patients and repetitive infections. J Infect. 2005; 50:34–40. PMID: 15603838.

2. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014; 37(Suppl 1):S81–S90. PMID: 24357215.
3. International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009; 32:1327–1334. PMID: 19502545.
4. Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997; 14:29–34. PMID: 9017350.

5. Muller LM, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AI, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis. 2005; 41:281–288. PMID: 16007521.

6. Tanaka Y. Immunosuppressive mechanisms in diabetes mellitus. Nihon Rinsho. 2008; 66:2233–2237. PMID: 19069085.
7. Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, et al. Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care. 2000; 23:187–191. PMID: 10868829.

8. Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003; 26:510–513. PMID: 12547890.

9. Infante-Cossío P, Fernández-Hinojosa E, Mangas-Cruz MA, González-Pérez LM. Ludwig's angina and ketoacidosis as a first manifestation of diabetes mellitus. Med Oral Patol Oral Cir Bucal. 2010; 15:e624–e627. PMID: 20173723.
10. Huang TT, Tseng FY, Yeh TH, Hsu CJ, Chen YS. Factors affecting the bacteriology of deep neck infection: a retrospective study of 128 patients. Acta Otolaryngol. 2006; 126:396–401. PMID: 16608792.

11. Chandu A, Macisaac RJ, Smith AC, Bach LA. Diabetic ketoacidosis secondary to dento-alveolar infection. Int J Oral Maxillofac Surg. 2002; 31:57–59. PMID: 11936401.

12. Huang TT, Tseng FY, Liu TC, Hsu CJ, Chen YS. Deep neck infection in diabetic patients: comparison of clinical picture and outcomes with nondiabetic patients. Otolaryngol Head Neck Surg. 2005; 132:943–947. PMID: 15944569.

13. Sim YB, Park SH, Kang YJ, Kim SM, Lee JK, Jung JS, et al. The regulation of blood glucose level in physical and emotional stress models: possible involvement of adrenergic and glucocorticoid systems. Arch Pharm Res. 2010; 33:1679–1683. PMID: 21052944.

14. Lippi G, Mattiuzzi C, Targher G. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010; 362:2030. author reply 2031. PMID: 20505184.

15. Alexander M, Krishnan B, Shenoy N. Diabetes mellitus and odontogenic infections: an exaggerated risk? Oral Maxillofac Surg. 2008; 12:129–130. PMID: 18575903.
16. Carton JA, Maradona JA, Nuño FJ, Fernandez-Alvarez R, Pérez-Gonzalez F, Asensi V. Diabetes mellitus and bacteraemia: a comparative study between diabetic and non-diabetic patients. Eur J Med. 1992; 1:281–287. PMID: 1341610.
17. Stoeckle M, Kaech C, Trampuz A, Zimmerli W. The role of diabetes mellitus in patients with bloodstream infections. Swiss Med Wkly. 2008; 138:512–519. PMID: 18792825.
18. Geerlings SE, Hoepelman AI. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol Med Microbiol. 1999; 26:259–265. PMID: 10575137.

19. Rao DD, Desai A, Kulkarni RD, Gopalkrishnan K, Rao CB. Comparison of maxillofacial space infection in diabetic and nondiabetic patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 110:e7–e12. PMID: 20656528.

20. Chang JS, Yoo KH, Yoon SH, Ha J, Jung S, Kook MS, et al. Odontogenic infection involving the secondary fascial space in diabetic and non-diabetic patients: a clinical comparative study. J Korean Assoc Oral Maxillofac Surg. 2013; 39:175–181. PMID: 24471039.

21. Zheng L, Yang C, Zhang W, Cai X, Kim E, Jiang B, et al. Is there association between severe multispace infections of the oral maxillofacial region and diabetes mellitus? J Oral Maxillofac Surg. 2012; 70:1565–1572. PMID: 22014938.

22. Koraćević G, Vasiljević S, Velicković-Radovanović R, Sakac D, Obradović S, Damjanović M, et al. Stress hyperglycemia in acute myocardial infarction. Vojnosanit Pregl. 2014; 71:858–869. PMID: 25282785.

23. Burekovic A, Dizdarevic-Bostandzic A, Godinjak A. Poorly regulated blood glucose in diabetic patients-predictor of acute infections. Med Arch. 2014; 68:163–166. PMID: 25568525.

24. Jupiter DC, Humphers JM, Shibuya N. Trends in postoperative infection rates and their relationship to glycosylated hemoglobin levels in diabetic patients undergoing foot and ankle surgery. J Foot Ankle Surg. 2014; 53:307–311. PMID: 24246477.

25. Halkos ME, Puskas JD, Lattouf OM, Kilgo P, Kerendi F, Song HK, et al. Elevated preoperative hemoglobin A1c level is predictive of adverse events after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 2008; 136:631–640. PMID: 18805264.

26. Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosenthal RA. Long-term glycemic control and postoperative infectious complications. Arch Surg. 2006; 141:375–380. PMID: 16618895.

27. Lee JK, Kim HD, Lim SC. Predisposing factors of complicated deep neck infection: an analysis of 158 cases. Yonsei Med J. 2007; 48:55–62. PMID: 17326246.

28. Rega AJ, Aziz SR, Ziccardi VB. Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. J Oral Maxillofac Surg. 2006; 64:1377–1380. PMID: 16916672.

29. Wills PI, Vernon RP Jr. Complications of space infections of the head and neck. Laryngoscope. 1981; 91:1129–1136. PMID: 7242204.

30. Beck HJ, Salassa JR, McCaffrey TV, Hermans PE. Life-threatening soft-tissue infections of the neck. Laryngoscope. 1984; 94:354–362. PMID: 6366410.

31. Zheng L, Yang C, Kim E, Zhang W, Cai X, Jiang B, et al. The clinical features of severe multi-space infections of the head and neck in patients with diabetes mellitus compared to non-diabetic patients. Br J Oral Maxillofac Surg. 2012; 50:757–761. PMID: 22349040.

32. Liu R, Desta T, He H, Graves DT. Diabetes alters the response to bacteria by enhancing fibroblast apoptosis. Endocrinology. 2004; 145:2997–3003. PMID: 15033911.

33. Naguib G, Al-Mashat H, Desta T, Graves DT. Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J Invest Dermatol. 2004; 123:87–92. PMID: 15191547.

34. Huang TT, Liu TC, Chen PR, Tseng FY, Yeh TH, Chen YS. Deep neck infection: analysis of 185 cases. Head Neck. 2004; 26:854–860. PMID: 15390207.

35. Sahly H, Podschun R, Ullmann U. Klebsiella infections in the immunocompromised host. Adv Exp Med Biol. 2000; 479:237–249. PMID: 10897425.

36. Leibovici L, Yehezkelli Y, Porter A, Regev A, Krauze I, Harell D. Influence of diabetes mellitus and glycaemic control on the characteristics and outcome of common infections. Diabet Med. 1996; 13:457–463. PMID: 8737028.

37. Gallacher SJ, Thomson G, Fraser WD, Fisher BM, Gemmell CG, MacCuish AC. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. 1995; 12:916–920. PMID: 8846684.

Table 1
Demographics and HbA1c levels for group 1 and group 2
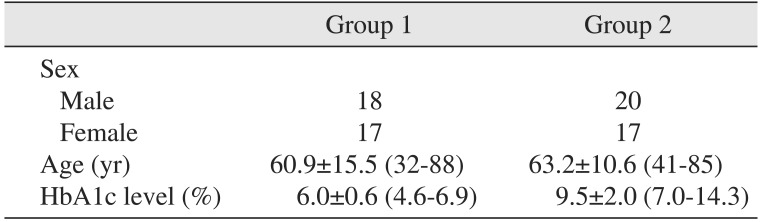
| Group 1 | Group 2 | |
|---|---|---|
| Sex | ||
| Male | 18 | 20 |
| Female | 17 | 17 |
| Age (yr) | 60.9±15.5 (32-88) | 63.2±10.6 (41-85) |
| HbA1c level (%) | 6.0±0.6 (4.6-6.9) | 9.5±2.0 (7.0-14.3) |
Table 2
Duration of hospitalization of group 1 and group 2
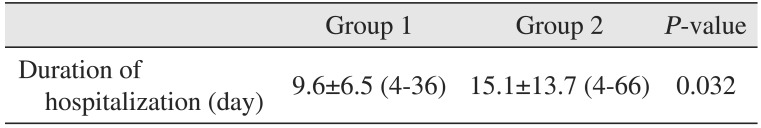
| Group 1 | Group 2 | P-value | |
|---|---|---|---|
| Duration of hospitalization (day) | 9.6±6.5 (4-36) | 15.1±13.7 (4-66) | 0.032 |
Table 3
Linear regression analysis between HbA1c level and hospitalization period
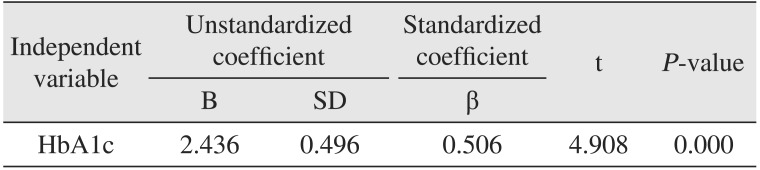
| Independent variable | Unstandardized coefficient | Standardized coefficient | t | P-value | |
|---|---|---|---|---|---|
| B | SD | β | |||
| HbA1c | 2.436 | 0.496 | 0.506 | 4.908 | 0.000 |
Table 4
Parameters of inflammation in group 1 and group 2
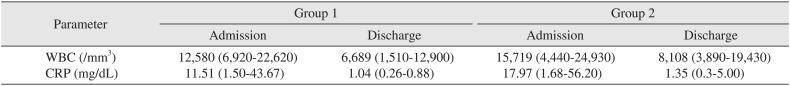
Table 5
Distribution of the involved fascial space
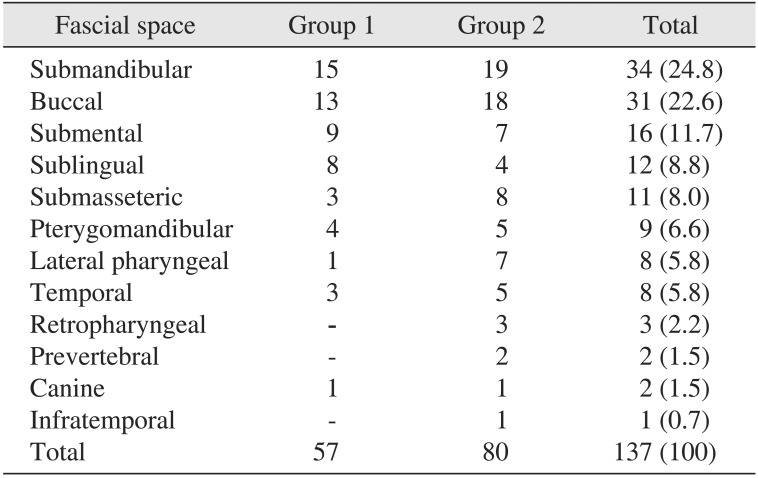
Table 6
Complications of maxillofacial fascial space infection
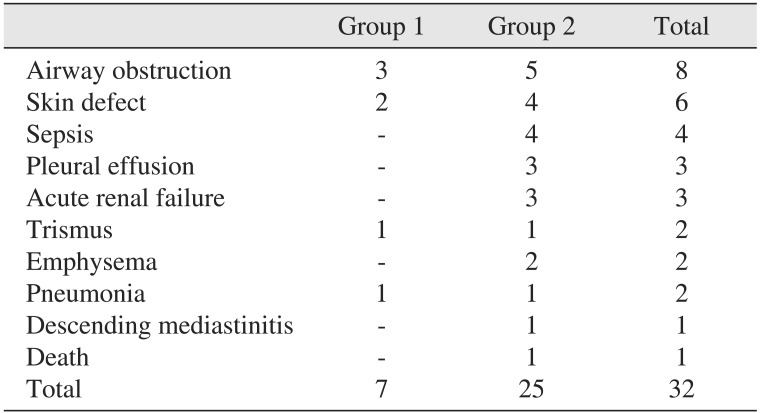
Table 7
Chi-square analysis of HbA1c level and complication between the two groups
| Complication | Group 1 | Group 2 | Relative risk | P-value |
|---|---|---|---|---|
| No | 29 (82.9) | 18 (48.6) | 3.005 | 0.002 |
| Yes | 6 (17.1) | 19 (51.4) |
Table 8
Logistic regression analysis between HbA1c level and complication

| Complication prevalence | |||
|---|---|---|---|
| Exp(B) | 95% CI | P-value | |
| HbA1c level (%) | 1.404 | 1.112-1.772 | 0.004 |
Table 9
Distribution of causative microorganisms




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download