This article has been
cited by other articles in ScienceCentral.
Abstract
Objectives
The increased prevalence of antibiotic resistance is an outcome of evolution. Most patients presenting with odontogenic space infections also have associated systemic co-morbidities such as diabetes mellitus resulting in impaired host defense. The present study aims to compare the odontogenic spaces involved, antibiotic susceptibility of microorganisms, length of hospital stay, and the infl uence of systemic comorbidities on treatment outcome in diabetic patients.
Materials and Methods
A 2-year prospective study from January 2012 to January 2014 was conducted on patients with odontogenic maxillofacial space infections. The patients were divided into two groups based on their glycemic levels. The data were compiled and statistically analyzed.
Results
A total of 188 patients were included in the study that underwent surgical incision and drainage, removal of infection source, specimen collection for culture-sensitivity, and evaluation of diabetic status. Sixty-one out of 188 patients were found to be diabetic. The submandibular space was the most commonly involved space, and the most prevalent microorganism was Klebsiella pneumoniae in diabetics and group D Streptococcus in the nondiabetic group.
Conclusion
The submandibular space was found to be the most commonly involved space, irrespective of glycemic control. Empiric antibiotic therapy with amoxicillin plus clavulanic acid combined with metronidazole with optimal glycemic control and surgical drainage of infection led to resolution of infection in diabetic as well as nondiabetic patients. The average length of hospital stay was found to be relatively longer in diabetic individuals.
Go to :

Keywords: Comparative analysis, Odontogenic, Diabetics, Infection
I. Introduction
Orofacial infections of odontogenic origin have long plagued mankind. The discovery of "the miracle drug" by Fleming in the year 1928 and the routine use of penicillin after the landmark discovery of the powder form of the antibiotic by Florey and Chain led to a significant change in the management of odontogenic space infections
1.
In an era of ever increasing use of antimicrobial therapy, odontogenic infections continue to be the most commonly encountered challenge by the maxillofacial surgeon. The spread of infection is governed by factors such as impaired host defense, the virulence of microorganism, functional abnormalities of the host, and a lack of or delayed treatment
2.
Diabetes mellitus is a metabolic syndrome characterized by absolute or relative insulin deficiency. Recurrent infections continue to be a systemic complication of diabetes and are thought to occur as a result of impaired host defense.
The microbiology of odontogenic infections in diabetic and nondiabetic individuals has been found to be variable. The effect of diabetes on infection severity, length of hospital stay, susceptibility to antibiotics, and outcome of treatment remains unanswered.
In light of this, a study was conducted to compare the odontogenic spaces involved, antibiotic susceptibility of microorganisms, length of hospital stay, and the outcome of treatment in diabetic versus nondiabetic individuals.
Go to :

II. Materials and Methods
A 2-year prospective study from January 2012 to January 2014 was carried out in patients who presented to our unit with maxillofacial space infections of odontogenic origin. A total of 188 patients were included in the study that underwent surgical drainage. they were divided into two groups based on their diabetic status; (1) group I: patients with fasting blood sugar levels greater than 130 mg/dL or with a known history of diabetes mellitus and (2) group II: patients presenting with maxillofacial infections of odontogenic origin and fasting sugar levels less than 130 mg/dL and no previous history of medical management of hyperglycemia.
Patients presenting with maxillofacial infections of nonodontogenic origin or those taking antibiotics prior to reporting to our unit were excluded from the study.(
Table 1) The study was conducted following approval by the Institutional Review Board of Goa Dental College and Hospital as per the Helsinki declaration. Written consent was obtained from patients that participated in the study.
Table 1
Inclusion criteria

|
Criterion |
Inclusion |
Exclusion |
|
Group I |
Patients with fasting blood sugar levels greater than 130 mg/dL or with a known history of diabetes mellitus |
Maxillofacial infections of nonodontogenic origin |
|
Group II |
Patients presenting with maxillofacial infections of odontogenic origin and fasting sugar levels less than 130 mg/dL with no previous history of medical management of hyperglycemia |
Patients taking antibiotics before reporting to our unit |

Microbial specimens were obtained from 188 patients either as swabs or by means of aspiration. The sample was cultured on blood or MacConkey's agar and inoculated at 37 degrees for 24 to 48 hours. Antibiotic sensitivity testing of isolated strains was carried out by the Kirby-Bauer disc diffusion method. The susceptibility tests were performed as per Clinical and Laboratory Standards Institute (CLSI) guidelines. The results were reported as sensitive, moderately sensitive, or resistant to the different antibiotics.
Both groups I and II were evaluated based on the following parameters: (1) space(s) involved, (2) organisms involved based on culture, (3) white blood cell (WBC) count, (4) sensitivity of organisms, (5) duration of hospital stay, (6) need to change empirical therapy, and (7) complications encountered.
The results were compiled and statistically analyzed using unpaired t-tests and chi-square tests. Correlations were considered statistically significant at P<0.05.
Go to :

III. Results
A total of 188 patients were included in the study; 108 patients (57.44%) were men and 80 patients (42.55%) were women. Group I included 61 patients and group II had 127 patients. The mean age in group I was 42.72 years and 57.89 years in group II.(
Table 2)
Table 2
Patient demographics
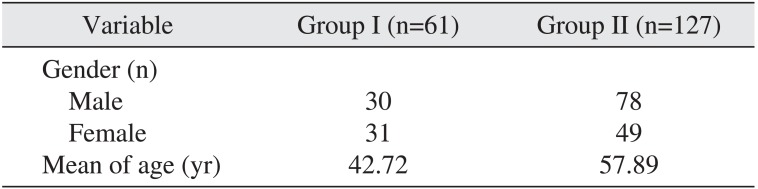
|
Variable |
Group I (n=61) |
Group II (n=127) |
|
Gender (n) |
|
|
|
Male |
30 |
78 |
|
Female |
31 |
49 |
|
Mean of age (yr) |
42.72 |
57.89 |

The most commonly involved space in both groups was the submandibular space followed by the buccal space. Ludwig's angina was seen in 3 patients (4.91%) in group I and 1 patient (0.78%) in group II. The diagnosis of involved space(s) was confirmed on clinical examination.(
Table 3)
Table 3
Spaces involved in diabetic and nondiabetic patients
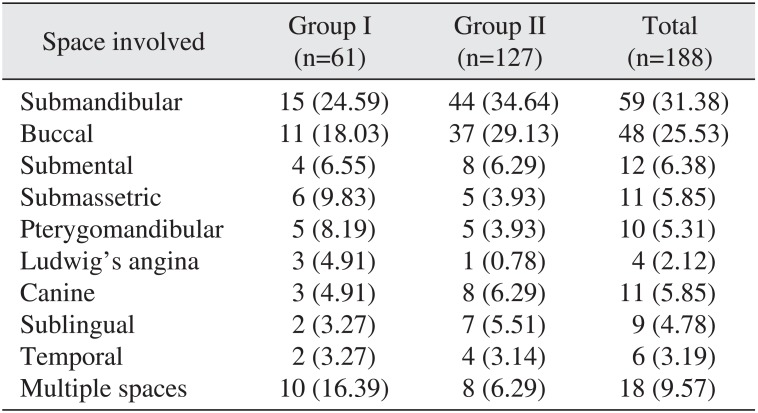
|
Space involved |
Group I (n=61) |
Group II (n=127) |
Total (n=188) |
|
Submandibular |
15 (24.59) |
44 (34.64) |
59 (31.38) |
|
Buccal |
11 (18.03) |
37 (29.13) |
48 (25.53) |
|
Submental |
4 (6.55) |
8 (6.29) |
12 (6.38) |
|
Submassetric |
6 (9.83) |
5 (3.93) |
11 (5.85) |
|
Pterygomandibular |
5 (8.19) |
5 (3.93) |
10 (5.31) |
|
Ludwig's angina |
3 (4.91) |
1 (0.78) |
4 (2.12) |
|
Canine |
3 (4.91) |
8 (6.29) |
11 (5.85) |
|
Sublingual |
2 (3.27) |
7 (5.51) |
9 (4.78) |
|
Temporal |
2 (3.27) |
4 (3.14) |
6 (3.19) |
|
Multiple spaces |
10 (16.39) |
8 (6.29) |
18 (9.57) |

Of the organisms isolated,
Klebsiella spp. was the most common bacteria in group I (24.59%) and group D
Streptococcus (29.13%) was the most common organism in group II.(
Table 4)
Table 4
Isolated organisms from diabetic and nondiabetic patients
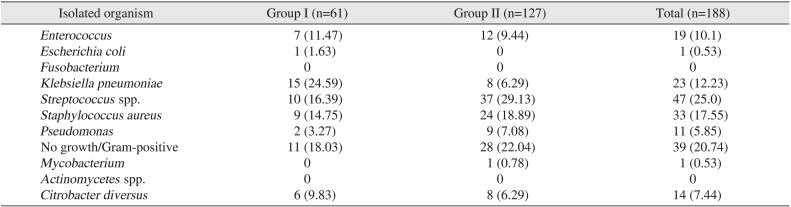
|
Isolated organism |
Group I (n=61) |
Group II (n=127) |
Total (n=188) |
|
Enterococcus
|
7 (11.47) |
12 (9.44) |
19 (10.1) |
|
Escherichia coli
|
1 (1.63) |
0 |
1 (0.53) |
|
Fusobacterium
|
0 |
0 |
0 |
|
Klebsiella pneumoniae
|
15 (24.59) |
8 (6.29) |
23 (12.23) |
|
Streptococcus spp. |
10 (16.39) |
37 (29.13) |
47 (25.0) |
|
Staphylococcus aureus
|
9 (14.75) |
24 (18.89) |
33 (17.55) |
|
Pseudomonas
|
2 (3.27) |
9 (7.08) |
11 (5.85) |
|
No growth/Gram-positive |
11 (18.03) |
28 (22.04) |
39 (20.74) |
|
Mycobacterium
|
0 |
1 (0.78) |
1 (0.53) |
|
Actinomycetes spp. |
0 |
0 |
0 |
|
Citrobacter diversus
|
6 (9.83) |
8 (6.29) |
14 (7.44) |

All patients were started on intravenous empirical therapy consisting of amoxicillin, clavulanic acid, and metronidazole at the time of presentation. The diabetic patients received insulin therapy on a sliding scale or as a fixed dose following consultation with the physician.
Resistance to empirical therapy was seen in 8 patients (13.11%) in group I and 5 patients (3.93%) in group II.
The hospital stay of patients in group I was found to be relatively longer as compared to those in group II, with a mean±standard deviation days of 9.6±8.16 days and 5.15±3.64 days, respectively (P=0.003).
The only significant hematologic finding on routine blood investigation in both groups was an elevated WBC count above 11,000 cells/mL (P=0.69).
The complications encountered were need for reexploration, extension of infection in the mediastinal compartment, and respiratory distress due to airway obstruction.
Go to :

IV. Discussion
Management of odontogenic maxillofacial infections is a challenging task for the surgeon, even in the era of antimicrobial therapy. Untreated patients often present to the dental office with involvement of multiple secondary spaces that can be life-threatening due to airway obstruction.
Diabetic patients are not only at high risk for developing infectious diseases, but they also respond poorly to infections once they occur, particularly in the context of suboptimal glucose control. Systemic hyperglycemia results in derangement of the immune system, including neutrophil function, cellular immunity, and complement function
34. This prospective study was designed to compare the anatomical spaces involved, microbiology and antibiotic sensitivity patterns, duration of hospital stay, and the need to change empiric therapy.
The source of maxillofacial infection can be infective, inflammatory, or traumatic. Of these, odontogenic causes are the most commonly encountered
567. The most common source of infection in our study was the mandibular first molar, which was seen in 102 patients (54.25%).
In agreement with other studies, diabetic patients also showed a higher incidence of involvement of multiple spaces at the time of presentation
8. The submandibular space was followed by the buccal space as the most commonly involved spaces, irrespective of glycemic status.
Knowledge of the potential spectrum of pathogens, as well as regional resistance status, is important for rational therapeutics.
The microbial spectrum of odontogenic space infections has been changing due to inadvertent use of antibiotics. The quest for an ideal antibiotic is still a dilemma for the clinician. The present study evaluated the bacteriology and antibiotic sensitivity patterns in both diabetic as well as nondiabetic individuals with the aim of informing necessary changes in the management.
A review of the literature suggests that the microbial fl ora in cases of maxillofacial infections is of mixed origin
910. The results of the present study did not exactly coincide with literature findings. Aerobic microorganisms were more predominant in group II patients whereas mixed fl ora was a predominant feature in group I patients.
A recent study by Rega et al.
11 suggests that aerobic organisms outnumbered anaerobic organisms by a ratio of 2:1. The results of the present study showed a variation in group I, where the predominant fl ora was anaerobic.
One reason that explains anaerobic predominance could be the delay in treatment initiation, during which anaerobes take over after utilization of oxygen by aerobic organisms.
WBC count is a useful indicator in assessing the improvement or regression of a patient's response to therapy, rather than predicting the actual patient status. In this study, the WBC count in both the diabetic and nondiabetic groups was greater than 11,000 cells/mL; the infection itself leads to increased WBC levels, irrespective of the underlying diabetic status
12.
Pyrexia at the time of presentation was more common in group I individuals as compared to patients in group II, a finding that was in unison with other studies. The literature also suggests a statistical correlation between raised body temperatures owing to decreased host response
13.
In this study, 20.74% of individuals were negative for any growth. Reasons for this could be due to anaerobic infections, collection of samples after antibiotic administration, and occasional loss of organisms during handling, transportation, and processing of the samples
5.
The failure to isolate anaerobic organisms had no bearing on treatment outcome, as most patients responded well to empirical antimicrobial therapy.
The use of a beta-lactam as an empiric antibiotic in spite of the apparent rise of in vitro resistance is common, and infections are still susceptible. However, addition of metronidazole into the regimen has also been preferred. Studies conducted to identify empiric antimicrobial therapy for odontogenic infections have stated the use of amoxicillin with metronidazole as one of the most effective regimens. This suggests that presently there is no need to subject patients to an additional antibiotic regimen.
The combination of amoxicillin and clavulanic acid along with metronidazole can work effectively in both diabetic and nondiabetic patients
14.
A combination of amoxicillin plus clavulanic acid and metronidazole was used in 82% of cases; 86.52% in diabetic and 85.50% in nondiabetic patients.
The complications encountered were the need for reexploration and respiratory difficulty, especially in cases of Ludwig's angina due to elevation of the fl oor of the mouth; all three cases were seen in group I patients.
The severity of infection depends on the number of spaces involved, airway compression, general health status, and associated systemic co-morbidities. Patients with greater infection severity usually require a longer hospital stay to achieve adequate glycemic control
15.
The mechanism by which diabetes predisposes an individual to infection is multifactorial. Defects in the immune system along with vascular abnormalities encountered in diabetic patients predispose them to deep fascial neck infections, necrotizing infections, and fungal infections
16.
A review of the literature suggests that the outcome of infection following satisfactory glycemic control shows no significant difference. The authors of this study are also of the opinion that infection response following adequate glycemic control was similar
17.
Resolution of infection and improvement in general health status can be achieved if basic principles of surgical drainage and antibiotic therapy are followed
18.
Go to :

V. Conclusion
In conclusion, this study aims to highlight the management protocol for diabetic patients presenting with odontogenic space infections. The corner stone of management of infections in the oral and maxillofacial region remains the same in diabetic and nondiabetic patients, i.e., source control, drainage, and adjunctive antimicrobial therapy.
The following conclusions can be drawn from the findings of the present study:
(1) The submandibular space was the most commonly involved in both groups irrespective of diabetic status. Ludwig's angina was seen in 3 patients (4.91%) in group I and 1 patient (0.78%) in group II. Involvement of multiple fascial spaces was more common in diabetic patients.
(2) Satisfactory resolution of infection was seen following surgical drainage with adjuvant antimicrobial therapy consisting of amoxicillin/clavulanic acid and metronidazole, provided diabetic patients had adequate glycemic control.
(3) The duration of hospital stay in diabetic patients was relatively longer as compared to nondiabetic patients, a fact that is due to adequate glycemic control rather than infection resolution.
(4) The organism most commonly isolated from pus samples of diabetic patients was Klebsiella spp., whereas group D Streptococci were isolated from pus samples of diabetic patients.
Contrary to popular belief, radical management of maxillofacial infections in diabetic patients is not necessary based on findings of the present study.
Go to :






