Abstract
Objectives
The objective of this study was to identify salivary and serum concentrations of interleukin (IL)-8, IL-6, and tumor necrosis factor alpha (TNF-α) in patients with oral lichen planus, oral leukoplakia, oral submucous fibrosis, and healthy controls.
Materials and Methods
Patients selected included 54 oral lichen planus (41 to 65 years), 50 oral leukoplakia (42 to 65 years), 51 oral submucous fibrosis (41 to 65 years), and 50 healthy controls (42 to 65 years). Oral lichen planus, oral leukoplakia, and oral submucous fibrosis cases were diagnosed using histopathological analysis. Salivary and serum cytokine concentrations were measured using enzyme-linked immunoassay kits in all subjects.
Results
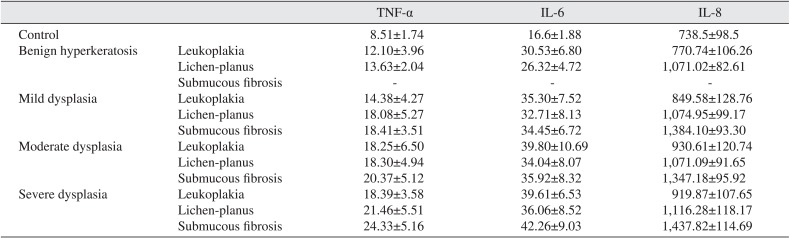
The levels of serum and salivary TNF-α, IL-6, and IL-8 were statistically significantly increased in oral leukoplakia, submucous fibrosis, and lichen planus in contrast to normal healthy subjects (P<0.05). Serum and salivary correlation analysis revealed strong and highly significant correlations for TNF-α, IL-6, and IL-8 in all groups (r=0.72-0.82, P<0.05).
Oral cancer is the eleventh most common cancer in the world1. Its incidence is predominantly high in northern India, other Asian countries, and in certain places in the western hemisphere. It has been reported that 90% of oral cancers in India among men were attributable to chewing and smoking habits. In India, the age-standardized incidence rate of oral cancer is 12.6 per 100,000 people2. Oral squamous cell carcinoma develops through a multi-step process of genetic, epigenetic, and metabolic changes resulting from exposure to carcinogens3. The initial presence of a precursor cell subsequently developing into cancer is well established in oral cancer4. Oral leukoplakia, submucous fibrosis, and lichen planus are major known precursor lesions. The prevalence of malignant transformation of oral lichen planus is around 0.5% and approximately 1% for leukoplakia5. Ability to clinically predict malignant transformation is difficult and routine histopathological diagnosis has limited prognostic value. Despite advanced technologies for early detection of oral precancerous and cancerous lesions, there are limitations for its use, including that diagnosis is essentially subjective, all lesions exhibiting dysplasia do not eventually become malignant and may even regress, and carcinoma can develop from lesions in which epithelial dysplasia was not diagnosed on previous biopsies67. Therefore, it is necessary to develop other methods for predicting the malignant potential of premalignant lesions and preventive measures7.
Recently, strong evidence has suggested that the nuclear factor-κB (NF-κB) signaling pathway plays a critical role in carcinogenesis, protection from apoptosis, and chemoresistance in a number of cancer types, including head and neck cancer, breast cancer, hepatocellular carcinoma, and gastric cancer89101112. Recently, accumulating evidence has suggested that the NF-κB-dependent cytokine levels are elevated in saliva and tissue specimens of patients with oral premalignant lesions13. Furthermore, different cytokines can act as diagnostic tools for detecting oral cancerous and precancerous lesions and conditions. Interleukin (IL)-6 levels are significantly increased in oral cancer patients14151617. It has been evident that different cytokines are expressed by cancerous cells, the most common of which include tumor necrosis factor alpha (TNF-α), IL-1, IL-6, and IL-8151617. Limited studies were conducted on roles of TNF-α, IL-6, and IL-8 in oral precancer and cancer151617. The local and systemic nature of these responses suggests the hypothesis that cytokines with proinflammatory and proangiogenic activity are produced by precancers and cancers and could contribute to the pathogenesis of oral malignancy. In this study, we hypothesized that salivary and serum TNF-α, IL-6, and IL-8 levels could be elevated in oral precancerous lesions and conditions. To test this hypothesis, this study was conducted to analyze the concentrations of TNF-α, IL-6, and IL-8 in oral precancer using saliva and serum samples.
CConsecutive patients clinically and histopathologically confirmed as having oral lichen planus lesions, oral leukoplakia, and oral submucous fibrosis were recruited for this study from Baba Nidhan Singh Hospital, Punjab, India, based on the definition of oral cancer and precancerous lesions by the World Health Organization9. Healthy normal patients served as the control group. Demographic characteristics of patient and controls characteristics were mentioned in Table 1. The mean and standard deviation of smoking and alcoholic status was also ascertained. Patients and healthy subjects with matched periodontal status were selected.
All subjects had neither a smoking history nor any visible oral lesions under careful examination. Additionally, they had not received any treatments for the oral lesions within 90 days prior to the specimen collection, and had no history, symptoms, or signs of systemic infections or other diseases.
Histopathological diagnosis was made by a single oral pathologist. This study was approved by the ethical committee of Baba Nidhan Singh Hospital, Punjab, India and informed consents were obtained from all subjects as per the Declaration of Helsinki.
For each sample, 1.0 mL of supernatant was used for the enzyme-linked immunoassay (ELISA) cytokine assay by using the human ELISA kit for TNF-α, IL-6, and IL-8 (R&D Systems Inc., Minneapolis, MN, USA), according to the manufacturer's instructions.
Blood and serum samples were drawn between 9:00 and 10:00 AM from all subjects. Serum was separated from blood cells by centrifugation at 1,000 g for 5 minutes. The whole unstimulated saliva was collected between 9:00 and 10:00 AM. The subjects abstained from eating and drinking for at least 2 hours prior to the sampling. All subjects were requested to swallow first, tilt their head forward at more than 45°, and then expectorate saliva (10 mL) into a sterile centrifuge tube without swallowing for 4 to 5 minutes. The saliva was then centrifuged for 25 minutes at 3,500 g, and the clarified supernatants were separated into 2.0 mL aliquots. All samples were immediately frozen at -60℃ for future use. Prior to assays, the serum or saliva supernatants were allowed to thaw completely at room temperature for two hours. For each sample, 1.0 mL of supernatant was used for the ELISA cytokine assays, using the human ELISA kit for TNF-α, IL-6, and IL-8, according to the manufacturer's instructions. The absorbances of the samples at 492 nm for TNF-α, IL-6, and IL-8 were measured with a spectrophotometer (Sirio; Seac, Florence, Italy). A standard curve was organized by plotting the absorbance value of the standards versus corresponding concentrations. The concentrations of the cytokines in the sample were then determined by extrapolating from the standard curve. Tests were run in duplicate to check reliability. Protein contents were expressed in pg/mL. The inter-assay coefficient of variation was 3.0% to 4.5% and the intra-assay coefficient of variation was 2.5% to 4.2%.
Data normality was evaluated by Smirnoff Kolmogorof's test. The Kruskal Wallis test was utilized for comparisons between the groups. Mann-Whitney test for comparisons performed to detect any differences between the two groups. A P<0.05 was considered as statistically significant. All data was statistically analyzed using the SPSS version 11.5 (SPSS Inc., Chicago, IL, USA).
The demographic characteristics of patient and controls are mentioned in Table 1. The levels of serum and salivary TNF-α, IL-6, and IL-8 were statistically significantly higher in advanced stages of precancerous lesions as compared to early stages (P<0.05).(Tables 2, 3) The levels of serum and salivary TNF-α, IL-6, and IL-8 were also statistically significantly increased in oral leukoplakia, submucous fibrosis, and lichen-planus in contrast to normal healthy subjects. Serum and salivary correlation analysis revealed a strong and highly significant correlation for TNF-α, IL-6, and IL-8 levels in all groups as shown in Table 4.
Serum and salivary TNF-α, IL-6, and IL-8 were higher in advanced stages as compared to early stages of precancerous lesions and conditions, data which supports previous studies1415161718192021222324. TNF-α, IL-6, and IL-8 are potent angiogenic meditators with significant effects on tumor growth, and are associated with increased tumor vessel density and a worsened outcome15161718. Thus, these cytokines might act as surrogate biomarkers of angiogenesis and prognosis. It has been previously reported that excessive cell proliferation and activation of cellular actions can be instigated by chronic inflammation, which leads to the induction of irreversible DNA damage25. TNF-α, IL-6, and IL-8 released through the inflammatory response would promote tumor growth, while tumor growth further stimulates the inflammatory response, resulting in cyclic progression26. In the present study, serum and salivary TNF-α, IL-6, and IL-8 levels were upregulated in precancerous lesions and conditions. This finding is in line with most previous conducted work14151617, although one study has shown that salivary TNF-α and IL-6 were downregulated1819. This could be due to a small sample size and not using age- or gender-matched samples. Proinflammatory cytokine levels are elevated in submucous fibrosis due to immunoregulatory activity. Increased levels of NF-κB mediators might be associated with the development of oral precancerous and cancerous lesions. In the normal cell, stimulation of cytokines causes growth inhibition, while in oral cancer cells, stimulation of cytokines leads to upregulation of positive cell cycle regulators including NF-κB, signal transducers, and activators of transcription and the mitogen-activated protein kinase/extracellular signal-regulated pathway16. Upregulation of TNF-α, IL-6, and IL-8 might be protective in action.
Smoking is a risk factor of oral precancerous and cancerous lesions2. However, while smoking and chronic alcohol usage might be risk factors for cancer, this study showed no significant difference between controls and patients. TNF-α might act as an endogenous tumor promoter as well as an inducer of tissue remodeling required for tumor growth and spread22. IL-6 signaling has also been implicated in tumorigenesis23. TNF-α, IL-6, and IL-8 were also elevated in periodontitis patients21, but in the present study, subjects with periodontitis were excluded. So, the present study shows that the elevation of TNF-α, IL-6, and IL-8 is correlated with precancerous and cancerous lesions. Indeed, elevation of these cytokines in saliva might indicate the potential precancerous development into oral cancer22. Alternatively, these cytokines may be improperly produced in precancerous lesions, which could lead to induced growth, invasion, disruption of tumor suppression, and immune status. NF-κB activation leads to the upregulation of anti-apoptotic genes as a cell survival mechanism, by inducing physiological stress, which triggers an inflammatory response. In addition, NF-κB induces cytokines that regulate TNF-α, IL-6, and IL-8, which leads to the recruitment of leukocytes to the sites of inflammation27. There was good correlation between salivary and serum TNF-α, IL-6, and IL-8 in all groups. Since saliva can be easily collected, measurement of these biomarkers of diseases may prove useful in early detection of oral cancer risks. The salivary analysis for oral diagnosis may prove a cost effective method for screening large populations24.
Hence, salivary levels of NF-κB mediators were significantly elevated and may have diagnostic and prognostic utility as useful biomarkers for detection of precancerous and cancerous lesions. This study did not take into account factors such as diet, alcoholic history, and environmental factors, so further studies are required on large samples including these variables to determine the relationship between salivary biomarkers and oral cancer, and to further clarify their mechanism of action. It can only be concluded that patients with oral precancerous lesions have elevated salivary and serum cytokines compared to healthy controls and that the levels of salivary cytokines increase with the severity of dysplasia.
References
1. Petersen PE. The world oral health report 2003. Geneva: World Health Organization;2003.
2. Petersen PE. The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003; 31(Suppl 1):3–23. PMID: 15015736.
3. Lippman SM, Hong WK. Molecular markers of the risk of oral cancer. N Engl J Med. 2001; 344:1323–1326. PMID: 11320393.

4. Reibel J. Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med. 2003; 14:47–62. PMID: 12764019.

5. Gupta PC, Bhonsle RB, Murti PR, Daftary DK, Mehta FS, Pindborg JJ. An epidemiologic assessment of cancer risk in oral precancerous lesions in India with special reference to nodular leukoplakia. Cancer. 1989; 63:2247–2252. PMID: 2720574.

6. Geum DH, Roh YC, Yoon SY, Kim HG, Lee JH, Song JM, et al. The impact factors on 5-year survival rate in patients operated with oral cancer. J Korean Assoc Oral Maxillofac Surg. 2013; 39:207–216. PMID: 24471047.

7. Rai B, Kaur J, Jacobs R. Direct tissue fluorescence imaging in relation to tissue, serum and salivary protoporphyrin for oral precancerous and cancerous lesions. Oral Oncol. 2011; 47(Suppl 1):S40.
8. Wang CY, Mayo MW, Baldwin AS Jr. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-kappaB. Science. 1996; 274:784–787. PMID: 8864119.
9. van der Waal I. Potentially malignant disorders of the oral and oropharyngeal mucosa; terminology, classification and present concepts of management. Oral Oncol. 2009; 45:317–323. PMID: 18674954.

10. Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammationassociated cancer. Nature. 2004; 431:461–466. PMID: 15329734.
11. Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004; 6:203–208. PMID: 15380510.
12. Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res. 2004; 119:139–173. PMID: 15164877.
13. Rhodus NL, Ho V, Miller CS, Myers S, Ondrey F. NF-kappaB dependent cytokine levels in saliva of patients with oral preneoplastic lesions and oral squamous cell carcinoma. Cancer Detect Prev. 2005; 29:42–45. PMID: 15734216.
14. Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003; 23:363–398. PMID: 12680238.
15. Piva MR, DE Souza LB, Martins-Filho PR, Nonaka CF, DE Santana Santos T, DE Souza Andrade ES, et al. Role of inflammation in oral carcinogenesis (part II): CD8, FOXP3, TNF-α, TGF-β and NF-κB expression. Oncol Lett. 2013; 5:1909–1914. PMID: 23833665.

16. Chang KP, Kao HK, Wu CC, Fang KH, Chang YL, Huang YC, et al. Pretreatment interleukin-6 serum levels are associated with patient survival for oral cavity squamous cell carcinoma. Otolaryngol Head Neck Surg. 2013; 148:786–791. PMID: 23426713.

17. Chen Z, Malhotra PS, Thomas GR, Ondrey FG, Duffey DC, Smith CW, et al. Expression of proinflammatory and proangiogenic cytokines in patients with head and neck cancer. Clin Cancer Res. 1999; 5:1369–1379. PMID: 10389921.
18. Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL. Interleukin-8 expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1995; 121:202–209. PMID: 7840929.

19. Rhodus NL, Cheng B, Myers S, Miller L, Ho V, Ondrey F. The feasibility of monitoring NF-kappaB associated cytokines: TNFalpha, IL-1alpha, IL-6, and IL-8 in whole saliva for the malignant transformation of oral lichen planus. Mol Carcinog. 2005; 44:77–82. PMID: 16075467.
20. St John MA, Li Y, Zhou X, Denny P, Ho CM, Montemagno C, et al. Interleukin 6 and interleukin 8 as potential biomarkers for oral cavity and oropharyngeal squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2004; 130:929–935. PMID: 15313862.

21. Brailo V, Vucićević-Boras V, Cekić-Arambasin A, Alajbeg IZ, Milenović A, Lukac J. The significance of salivary interleukin 6 and tumor necrosis factor alpha in patients with oral leukoplakia. Oral Oncol. 2006; 42:370–373. PMID: 16324876.

22. Frodge BD, Ebersole JL, Kryscio RJ, Thomas MV, Miller CS. Bone remodeling biomarkers of periodontal disease in saliva. J Periodontol. 2008; 79:1913–1919. PMID: 18834246.

23. Burke F, Relf M, Negus R, Balkwill F. A cytokine profile of normal and malignant ovary. Cytokine. 1996; 8:578–585. PMID: 8891439.

24. Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005; 41:2502–2512. PMID: 16199153.

25. Rai B, Kaur J, Jacobs R, Singh J. Possible action mechanism for curcumin in pre-cancerous lesions based on serum and salivary markers of oxidative stress. J Oral Sci. 2010; 52:251–256. PMID: 20587949.

27. Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS, Milroy R. The systemic inflammatory response, weight loss, performance status and survival in patients with inoperable non-small cell lung cancer. Br J Cancer. 2002; 87:264–267. PMID: 12177792.

Table 1
Demographic characteristics of patients and controls
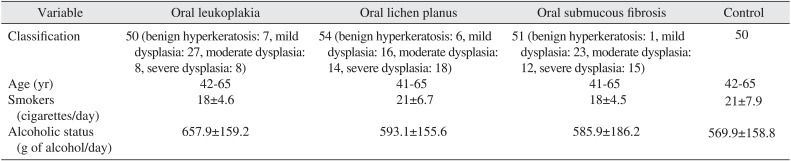
Table 2
Serum levels of NF-κB mediators in leukoplakia, lichen, submucous fibrosis patients and healthy controls
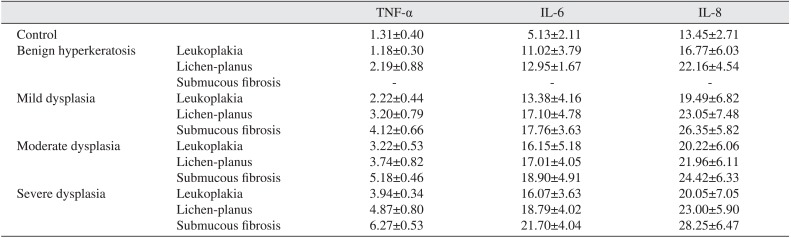
Table 3
Salivary levels of NF-κB mediators in leukoplakia, lichen, submucous fibrosis patients and control healthy




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download