Abstract
Objectives
This study was performed to analyze the cumulative survival rate of Osstem implants (Osstem Implant Co., Ltd.) over a seven-year period.
Materials and Methods
A total of 105 patients who had 467 Osstem implants that were placed at the Section of Dentistry, Seoul National University Bundang Hospital (Seongnam, Korea) from June 2003 through December 2005 were analyzed. The life table method and a cross-tubulation analysis, log rank test were used to evaluate the survival curve and the influence that the prognostic factors. The prognostic factors, i.e., age and gender of patients, diameter and length, type of implants, bone graft history and loading time were determined with a Cox proportional hazard model based on logistic regression analysis.
Albrektsson et al.1 defined the criteria for success in implantology as follows: no mobility, pain, or peri-implant radiolucency. They also state in their definition that peripheral bone loss for the first year should be less than 1 mm and should subsequently be no more than 0.2 mm.
However, there are cases where further peripheral bone loss and peri-implantitis occur, but pathologic bone loss does not progress when an appropriate treatment is applied, and the implant can still handle a functional load. Such instances should not be defined as failures but rather as surviving implants2.
Survival and success rates have increased with the introduction of the rough-surface implant, which improves osseointegration3,4,5. In addition, with the use of screw-type and tapered implants, bone density has improved by intraosseous compaction; in this case, when biting force is applied, the stress is evenly dispersed into the peripheral bone tissues6,7.
Rocci et al.8 reported that 2-3 years after machined surface implants were placed, 88% survived, whereas Lozada9 obtained a 95% success rate after an 8-year follow-up of hydroxyapatite-coated implants. Brechter et al.10 followed up patients for 30 months and obtained a survival rate of 98.5% for Ti-Unite (Nobel Biocare AG, Göteborg, Sweden) implants that had an anodizing oxidation surface. In contrast, Bornstein et al.11 included a 6-week healing period after placement of an implant that had been sandblasted and given an acid-etched surface treatment, and obtained a 99.03% success rate at 3 years. According to a prospective study examining the stability of tapered resorbable blasting media (RBM) surface implants in the posterior maxilla, the 1-year survival rate was 97.4% and the success rate was 94.7%12.
However, the success and the survival rates of implants is determined not only by characteristics such as the structure and surface treatment of the implants but also by host factors such as age, gender, systemic disease, tobacco use or dental hygiene; biological factors such as bone disease, bone mass and bone grafting; and prosthetic factors such as the form of the prosthetic, occlusion and loading period13.
Romanos and Nentwig14 performed comparative studies of implants with immediate loading and implants with delayed loading, and reported survival rates of 94.9% and 91.68%, respectively. Becktor et al.15 found a significant difference in implant survival rate between patients who had a bone graft on the maxillary edentulous jaw and those who did not. They reported survival rates of 75.1% and 84.0%, respectively.
Shumaker et al.16 noted that if proper management, such as a regular clinical assessment, plaque control, and mouth hygiene, is not followed, inflammatory lesions such as peri-implantitis can occur despite aggressive treatment.
The purpose of this study was to evaluate the 7-year cumulative survival rate of the Osstem implants, US II, US III, SS II, and GS II (Osstem Implant Co., Ltd., Busan, Korea), with various structure and surface treatments in order to confirm their stability after placement and to analyze the factors that are involved in implant survival.
The subjects of the study were patients who had Osstem implants that were placed at the Section of Dentistry, Seoul National University Bundang Hospital (Seongnam, Korea) from June 2003 through December 2005. One oral and maxillofacial surgeon and prosthodontist performed the treatment and we included patients who used tobacco as well as those with systemic disease. The study was conducted under Institutional Review Board approval of Seoul National University Bundang Hospital (B-1005-100-101). A total of 105 patients (55 male, 50 female) with a total of 467 Osstem implants were selected, and we examined their clinical records that contained historical charts and radiography. The observation period was between 42 and 83 months and began after placement of the final prosthesis.
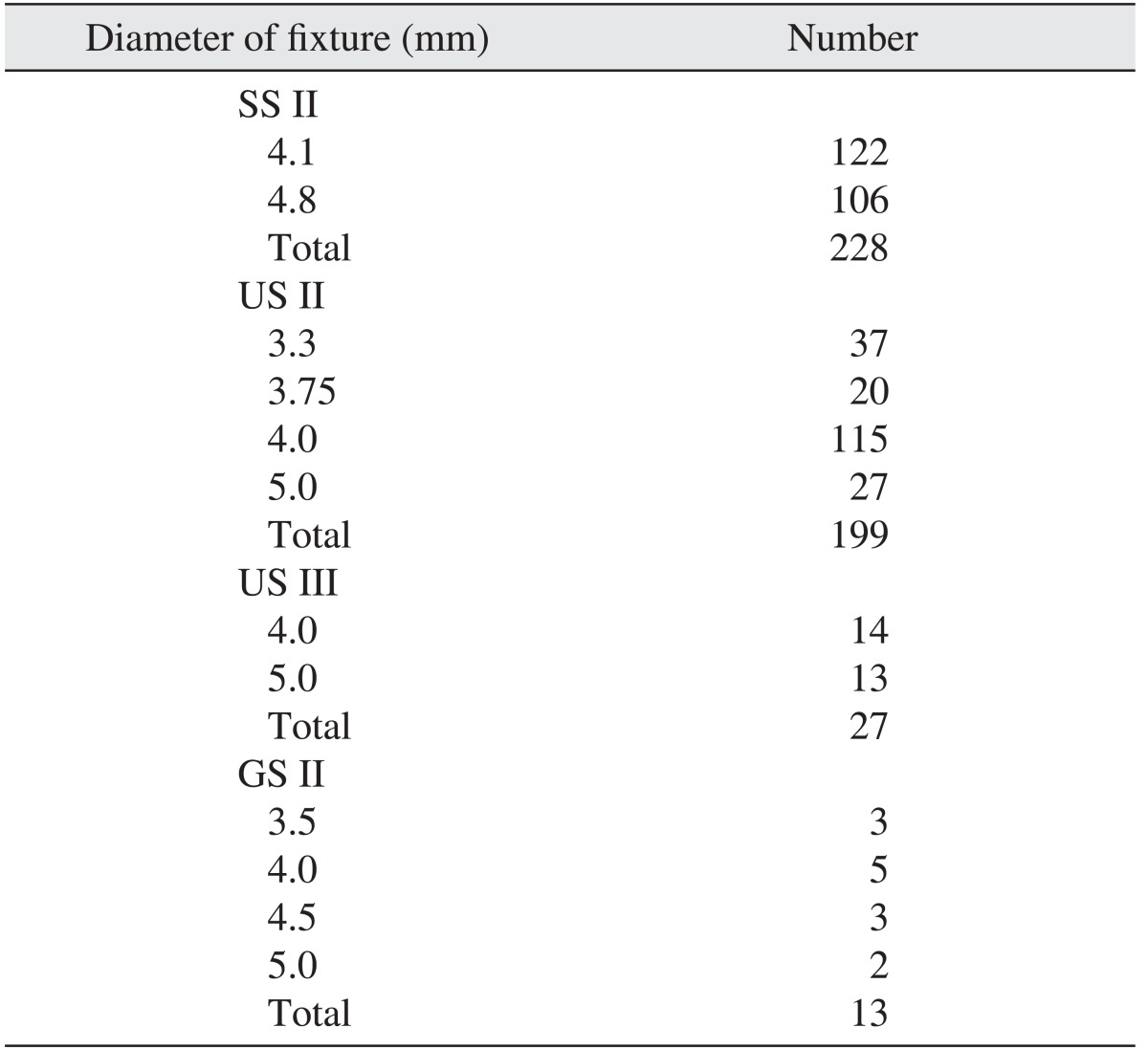
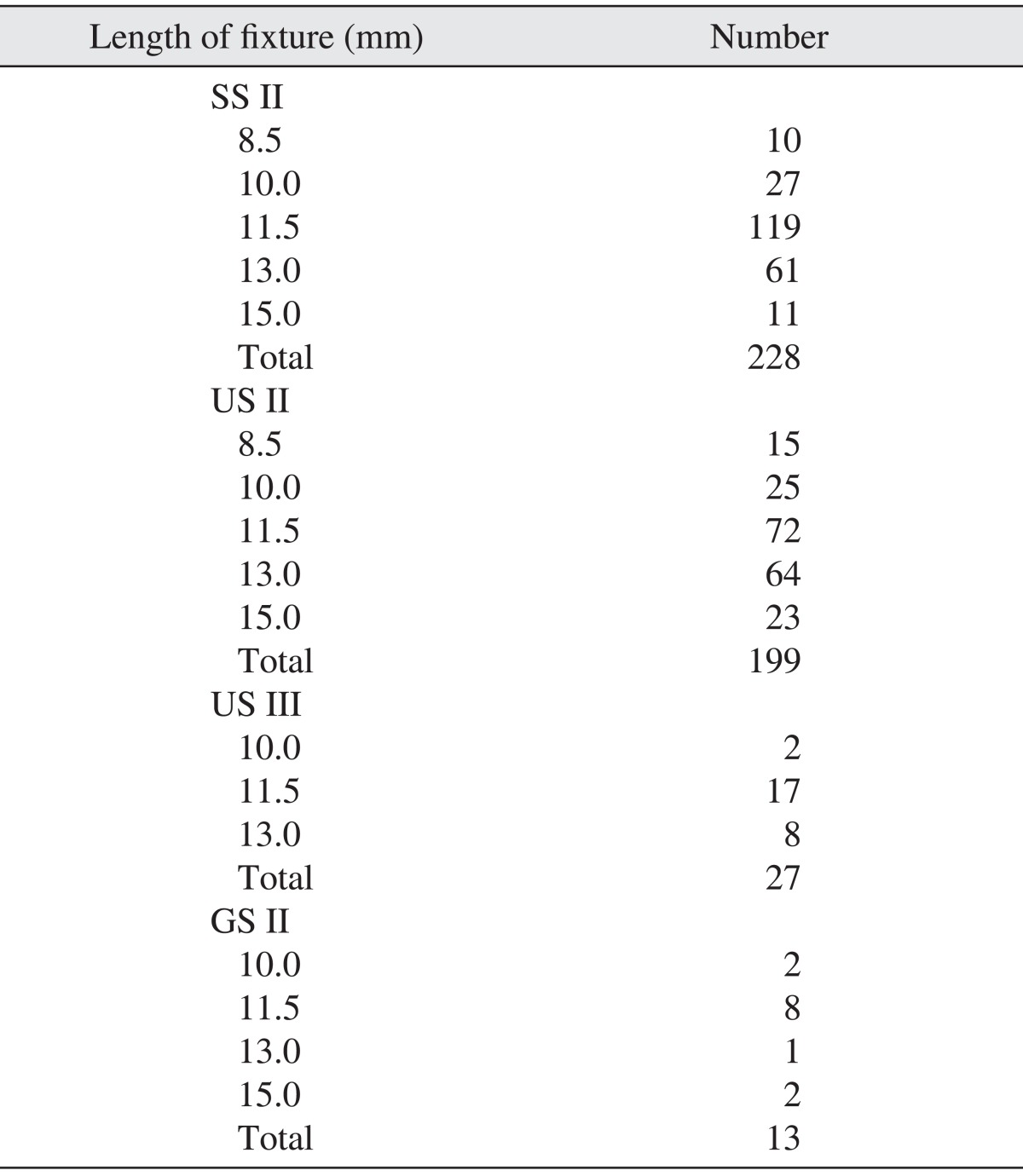
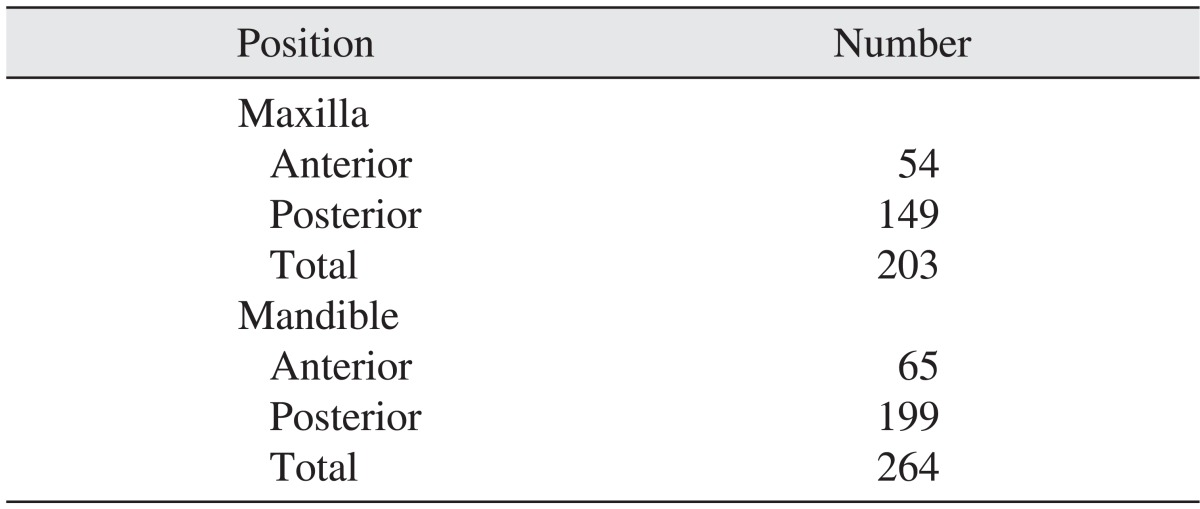
The implants were divided into 4 types: SS II (straight body, internal connection type, non-submerged type, RBM surface), US II (straight body, external connection type, RBM surface), US III (tapered body, external connection type, RBM surface), and GS II (straight body, internal connection submerged type and anodizing surface). Of these 4 types, 228, 199, 27, and 13 implants were respectively placed. For the US II implants, the implant of 4.0 mm in diameter was used most often, at 115 times; for the SS II implants, the 4.1 mm implant was used 122 times and the 4.8 mm implant was used 106 times.(Table 1) Implants of 11.5 mm in length were used most frequently in all product groups.(Table 2) In terms of positioning, 54 implants were placed in the anterior maxilla, 149 implants in the posterior maxilla, 65 implants in the anterior mandible and 199 implants in the posterior mandible.(Table 3)
Accompanying bone grafts included a sinus bone graft, a ridge augmentation and a guided bone regeneration (GBR). There were 337 implants that were placed with bone grafts and 130 implants that were placed without bone grafts. In total, 158 implants were placed using the one-stage (non-submerged) method, and 309 implants were placed using the two-stage (submerged) method.
In this study, we defined the conventional loading period for the lower jaw as 3 months and that for the upper jaw as 6 months; early loading and delayed loading were defined before and after the conventional loading period, respectively. The period before prosthesis loading ranged from 1 to 42 months and averaged 8.93±7.35 months. For the upper jaw, the period before prosthesis loading ranged from 1 to 41 months, with an average of 10.35±7.25 months, whereas these values for the lower jaw were 1 to 42.17 months, with an average of 7.81±7.26 months.
In determining prognosis for survival analysis, the following outcomes were regarded as failures: implant mobility, pain on percussion, presence of radiolucency around the implant on radiography and crestal bone loss of 1 mm or more over a period of one year2. Periapical radiography was performed immediately after implantation, and these radiographs were compared with annual X-rays; we measured the crestal bone loss in the mesial and distal aspects and calculated the average.
In this study, we used the life table method and a cross-tabulation analysis to analyze the cumulative survival rate of all 467 implants. We used the log rank test to evaluate the survival curve, and the influence that the prognostic factors, i.e., patient age, gender, bone graft history, implant diameter and width, loading time, implant type, and one-stage/two-stage implant, had on the survival rate was determined with a Cox proportional hazard model based on logistic regression analysis. Events were defined as cases in which implants were removed because of failure, and censored cases included those in which the implants survived until the observation concluded and those in which the patients were lost to follow-up or dropped out. We used PASW Statistics 18.0 for Windows (IBM Co., Armonk, NY, USA) as our statistics tool and the 95% confidence level to test the significance.
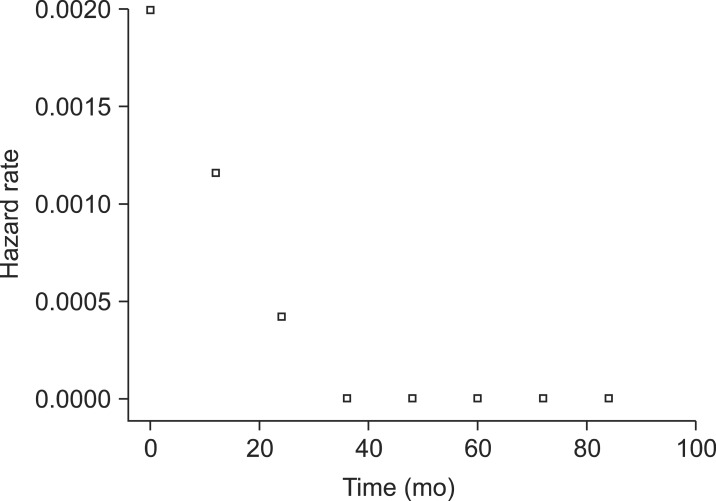
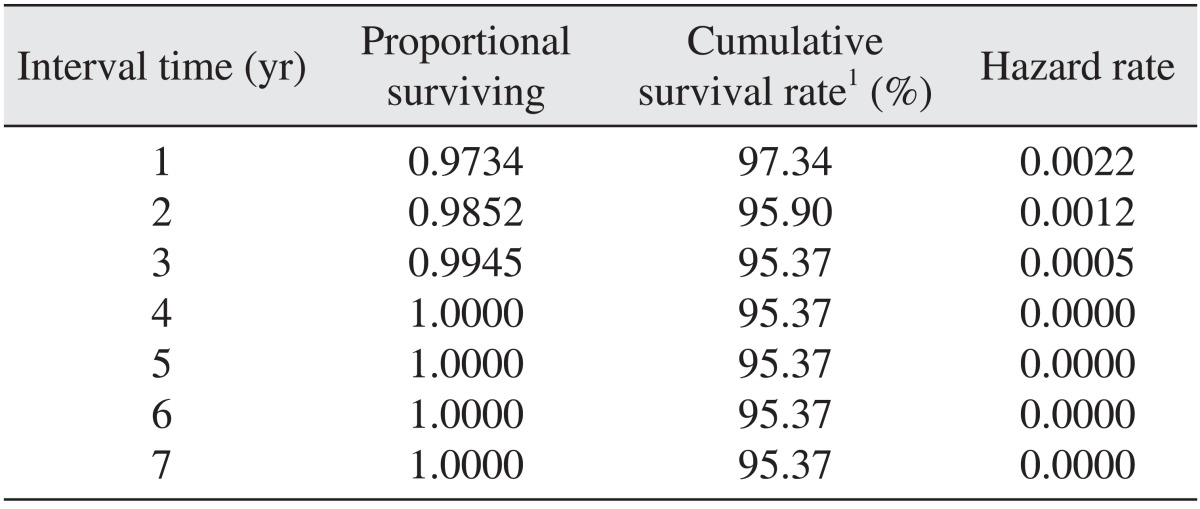
Of all 467 implants, 20 implants were removed because of failure. Removal occurred as follows: 1 implant was removed 2 months after the surgery, 3 implants after 3 months, 4 implants after 4 months, 2 implants after 5 months, 1 implant after 6 months, 1 implant after 9 months, 1 implant after 13 months, 3 implants after 14 months, 2 implants after 15 months, and 2 implants after 29 months. The cumulative survival rate for each interval was 97.34% at 1 year, 95.90% at 2 years, and 95.37% at 3-7 years, and the final cumulative survival rate was 95.37%.(Table 4, Figs. 1, 2)
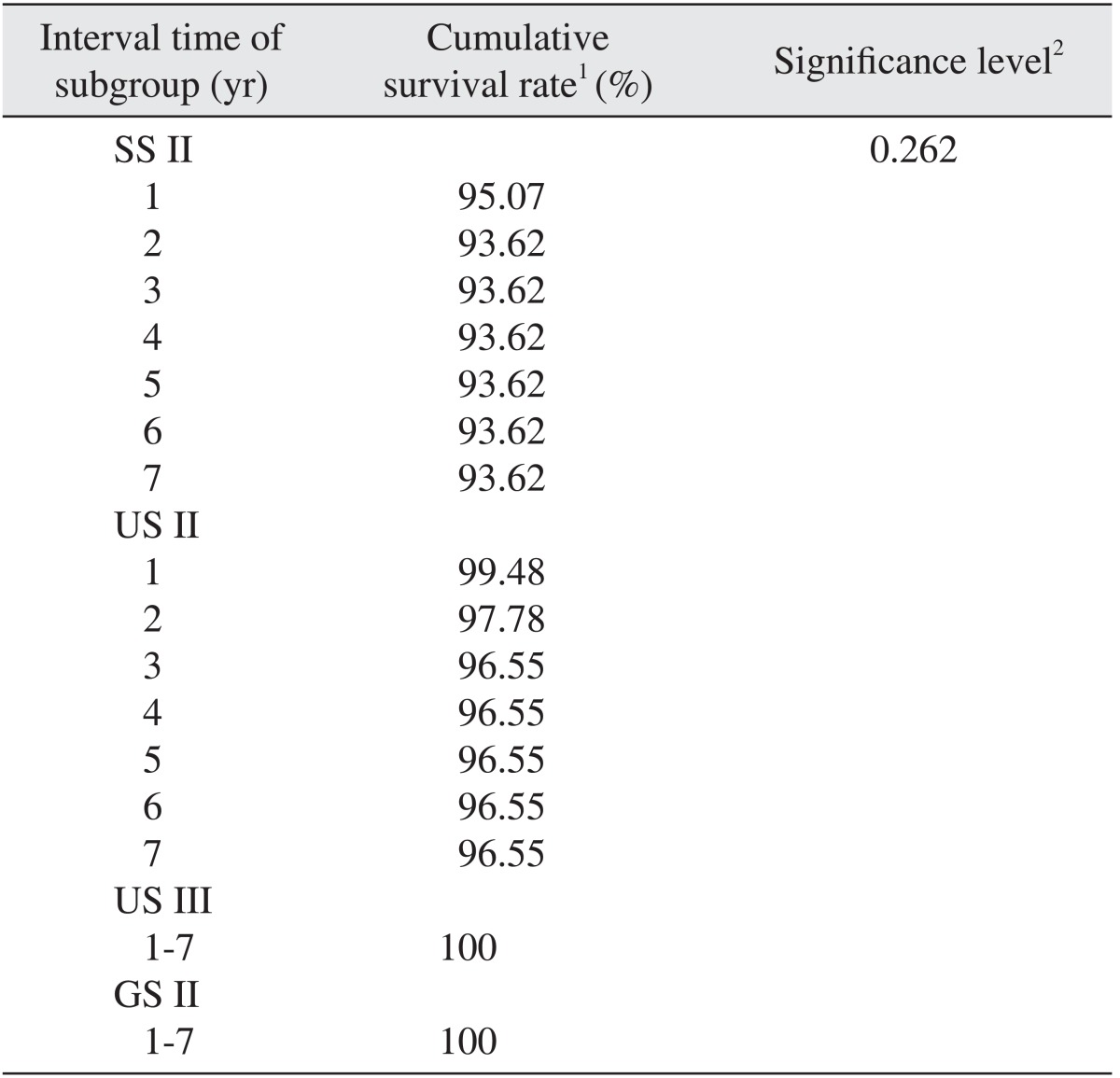
In groups US III and GS II, 100% of the implants survived. In group SS II, the cumulative survival rate for each interval was 95.07% at 1 year and 93.62% at 2-7 years, and the final cumulative survival rate was 93.62%. In group US II, the cumulative survival rates for each interval were 99.48% at 1 year, 97.78% at 2 years and 96.55% at 3-7 years, and the final cumulative survival rate was 96.55%. In a comparison of the cumulative survival rate for each group using cross-tabulation analysis (P>0.05) and logistic regression analysis (P>0.05), we found that there was no statistically significant difference.(Table 5, Fig. 3)
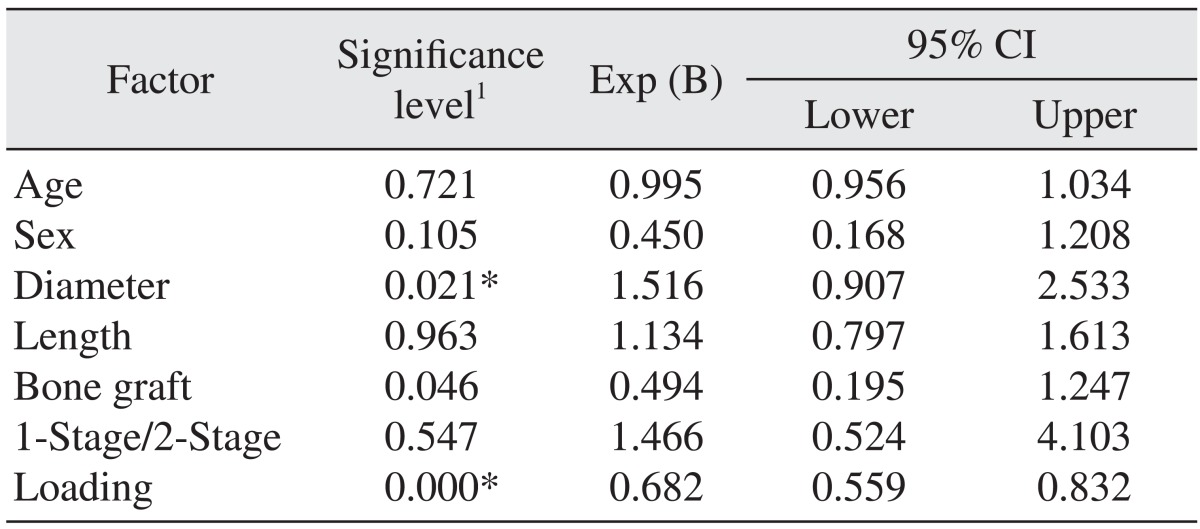
The cumulative survival rate was related to the diameter of the implant, the bone graft history and the loading period. Increased implant diameter, bone grafting, and a shorter healing time were factors that increased the risk of failure.(Table 6) In this study, among the 467 implants, the failure rate of implants placed in the maxilla was higher than for those placed in the mandible. In addition, 17 implants failed in cases that were accompanied by surgery with GBR. We applied early loading to 18 implants, regular loading to 210 implants and delayed loading to 239 implants. Of the 20 failed implants, 7 implants had early loading, 10 implants had regular loading and 3 implants had delayed loading. Ten failed implants occurred in two months of loading in one patient. The failure rate of early-loaded implants was 50% in the mandible and 87.5% in the maxilla. Defining 'wide-type' implants as those with diameters ≥4.8 mm17, 10 of the 20 implant failures were wide-type implants and 10 implants were standard implants.(Table 7)
The survival of implants placed in an edentulous jaw is influenced by implant characteristics, biological environment of the host and prosthetic restoration; implant survival has improved as implant structure, surface treatment and bone graft techniques have improved. Cavallaro17 reported that when 176 implants were placed on a normal alveolar bone, the survival rate at 3 years was 98.6%, and when 28 implants were immediately placed in the wound of the tooth extraction, the survival rate was 96.4%. Standford et al.18 placed 1,246 implants and analyzed the cumulative survival rate for a year after prosthetic loading (98.6%). Stafford19 revealed that when implantation occurred immediately after tooth extraction, the survival rate was 95.5%.
Survival analysis is a statistical technique in which the survival period is analyzed using a survival function, and the distribution of the survival period of a group, including members whose observation has not been completed, can be determined. In this study, the cumulative survival rates of Osstem implants were 97.34% at 1 year after placement, 95.90% after 2 years, and 95.37% after 3 years, which were not largely different from previous studies. According to a multicenter clinical study of Osstem implants after maxillary sinus floor elevation, the survival rates of US II and SS II implants were 99.2% and 95.8%20. A multicenter clinical study of Osstem GS II implants reported that the success and survival rates of the implants were 93.23% and 95.83%, respectively, in type IV bone21.
Failure of the implant in the early stage is related the primary stability of the implant, which can be improved by selecting implants with appropriate structure and surface properties as well as by improving the surgical method22. Osstem implants include implants with various structures depending on the gingival biotype, bone quality and bone defects; in the US III and GS II groups, 100% of implants survived; in the US II group, the final cumulative survival rate was 96.55%; and in the SS II group, the final cumulative survival rate was 93.62%.
Statistical analysis for the cumulative survival rate was performed using cross-tabulation (P>0.05) and logistic regression (P>0.05) analyses, but there were no statistically significant differences between any of the groups. However, because the numbers of followed US III and GS II implants were substantially smaller than those of the SS II and US II implants and because 100% of the US III and GS II implants survived, a comparative analysis on the differences between the SS II and US II implants is required.
The cross-tabulation and logistic regression analyses revealed no statistically significant differences between the SS II and US II implants. The US II implant has an external hex connection structure and is of the submerged type; because of the RBM surface treatment to improve the affinity with bone, this implant can easily be placed in various bone qualities and has outstanding holding power. In contrast, the SS II implant has an internal 8 degree morse taper and is of the non-submerged type; it has a stable connection structure and can be easily placed in various bone qualities. The GS II implant has an internal hex connection structure and is of the submerged type; its advantage is a dual micro and macro thread that minimizes bone resorption and provides optimal stress dispersion. The US III implant has an RBM surface and is of the tapered type with an external connection structure. When placed on a site with weak bone quality, powerful bone compression can be obtained that is effective at 1/3 of the retainer23,24.
Of the many factors that may influence the survival of implants, we confirmed that patient age and gender as well as the length of the implants, choice of one-stage method or two-stage method, and the type of product were not related to increased risk. Although it has been suggested that failure rate increases as age increases because of decreased bone density caused by bone resorption exceeding osteogenesis25, there was no increased risk related to age in this study as well as in the study of Smith et al.26. There was no difference in risk between males and females, as previously determined by Heberer et al.27. Renouard and Nisand28 reported that, by avoiding countersink or under-preparation at sites where the bone substance was poor, there was no difference in the success rate between rough-surface implants that were greater than 10 mm and less than 10 mm. In the present study, we also did not identify any risk related to implant length.
Some scholars have claimed that having a bone graft history was not a risk factor for implant failure29,30. However, a more serious surgical wound is more likely to affect the peripheral blood supply and therefore have a harmful influence on the healing of soft tissue and bone tissue. Becktor et al.15 found that the implant survival rates were 75.1% and 84.0% depending on whether or not there was a bone graft on the maxillary edentulous jaw, respectively, which represented a significant difference. Some authors have suggested that an implant placed in an adequate recipient site should perform better than an implant placed in a reconstructed site31. In this study, a history of bone grafting was directly correlated with increased risk, and we suggest that surgical techniques should focus not only on survival, but also on procedures that are less complicated, less invasive and are accompanied by smaller complications.
In addition, risk of failure increased with increasing implant diameter and earlier loading period. Ivanoff et al.32 reported an increased failure rate for implants with a wide diameter (≥5 mm) and attributed this effect not only to the difference in surgical technique, but also to the inability to obtain primary stability due to poor bone quality, which resulted in the use of wide implants as 'rescue' implants. Wide-diameter implants are often used when the bone substance and bone mass are poor, which increases the early failure rate.
Optimal loading time for upper dental prostheses after implant placement is still debated. The 2002 World Congress Consensus Meeting in Barcelona defined immediate loading as 3-4 days after surgery33. In addition, the 2003 ITI Consensus Conference defined the period within first 2 days after placement as immediate loading, the period between 48 hours and 3 months as early loading and the period between 3 months and 6 months as conventional loading34. At the 2008 ITI conference35, immediate loading referred to less than 1 week after placement, early loading referred to between 1 week and 2 months after placement and conventional loading referred to 2 months or more after placement. Under the same conditions, many other clinical studies as well as histomorphometric observations have confirmed better prognosis for delayed loading than for early loading30. In this study, similar to past findings, we observed that the risk of failure decreased as the loading time was delayed.
Of the 20 cases in which the implants were removed due to failure, 1 case was attributed to a psychiatric cause, and the remaining 19 were caused by the failure of osseointegration. Out of the 20 failed implants, 50% occurred in 1 patient. This patient had controlled DM as well as severe maxillary alveolar bony deficiency; therefore, he underwent interpositional bone grafting using iliac bone, and delayed implantation took place 4 months following the bone graft. Extensive bone grafting and surgical wounds are considered to be major factors in implant failure; the concentration of implant failures in one patient has been called 'clusterization' in previous studies36,37.
We suggest that the cause for this clusterization likely involved an immunological reaction of the patient in response to the systemic disease, the overloading of the bone substance or insufficient bone mass due to oral parafunction such as bruxism, the use of a difficult surgical technique or the application of early loading. In addition, of the 20 failed implants, 14 implants were SS II and 6 implants were US II; of the 6 US II implants, 5 implants occurred in the aforementioned patient.
One limitation of this study is that the factors that influenced implant survival were broad because we chose a retrospective study design. This approach limited our scope of research, and we did not consider the prosthetic aspect. In this study, we only considered the patient's age, gender, bone graft history, implant diameter and width, product type, one-stage/two-stage implant, and loading time as factors that influenced survival of implants. In addition to these factors, other factors such as the bone graft material, membrane, systemic disease, surgery time, type of anesthesia, type of prosthesis, condition of the occluding dentition, parafunction, and tobacco use also influence the survival rate, but were excluded in this study.
If we consider that each variable is interactional, then a duplicate statistical model, such as multi-regression analysis, would be required. However, because of the characteristics of the data, which have more discrete variables than continuous variables, we determined that it would be difficult to obtain a satisfactory result with this method. Therefore, we used cross-tabulation analysis and logistic regression analysis instead, which are widely used. A prospective study in the future will eliminate factors that influence the cumulative survival rate of implants and their prognosis while examining each factor independently.
The seven-year cumulative survival rate of Osstem implants was 95.37%. As the healing period after implant placement increased, the failure risk decreased. However, as the diameter of the implants increased, the failure risk also increased, depending on bone graft history and early loading.
References
1. Albrektsson T, Zarb G, Worthington P, Eriksson AR. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Implants. 1986; 1:11–25. PMID: 3527955.
2. Arisan V, Bölükbaşi N, Ersanli S, Ozdemir T. Evaluation of 316 narrow diameter implants followed for 5-10 years: a clinical and radiographic retrospective study. Clin Oral Implants Res. 2010; 21:296–307. PMID: 20443792.
3. Esposito M, Grusovin MG, Coulthard P, Thomsen P, Worthington HV. A 5-year follow-up comparative analysis of the efficacy of various osseointegrated dental implant systems: a systematic review of randomized controlled clinical trials. Int J Oral Maxillofac Implants. 2005; 20:557–568. PMID: 16161740.
4. Cochran DL. A comparison of endosseous dental implant surfaces. J Periodontol. 1999; 70:1523–1539. PMID: 10632528.

5. Nasatzky E, Gultchin J, Schwartz Z. The role of surface roughness in promoting osteointegration. Refuat Hapeh Vehashinayim. 2003; 20:8–19. 98PMID: 14515625.
6. Summers RB. A new concept in maxillary implant surgery: the osteotome technique. Compendium. 1994; 15:152154–156. 158 passimquiz 162. PMID: 8055503.
7. Rosenquist B, Grenthe B. Immediate placement of implants into extraction sockets: implant survival. Int J Oral Maxillofac Implants. 1996; 11:205–209. PMID: 8666452.

8. Rocci A, Martignoni M, Gottlow J. Immediate loading of Brånemark System TiUnite and machined-surface implants in the posterior mandible: a randomized open-ended clinical trial. Clin Implant Dent Relat Res. 2003; 5(Suppl 1):57–63. PMID: 12691651.

9. Lozada JL. Eight-year clinical evaluation of HA-coated implants. J Dent Symp. 1993; 1:67–69. PMID: 8186844.
10. Brechter M, Nilson H, Lundgren S. Oxidized titanium implants in reconstructive jaw surgery. Clin Implant Dent Relat Res. 2005; 7(Suppl 1):S83–S87. PMID: 16137092.

11. Bornstein MM, Lussi A, Schmid B, Belser UC, Buser D. Early loading of nonsubmerged titanium implants with a sandblasted and acid-etched (SLA) surface: 3-year results of a prospective study in partially edentulous patients. Int J Oral Maxillofac Implants. 2003; 18:659–666. PMID: 14579953.
12. Kim YK, Kim SG, Kim JH, Yi YJ, Yun PY. Prospective study of tapered resorbable blasting media surface implant stability in the maxillary posterior area. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012; 114:e19–e24. PMID: 22732852.

13. el Askary AS, Meffert RM, Griffin T. Why do dental implants fail? Part I. Implant Dent. 1999; 8:173–185. PMID: 10635160.

14. Romanos GE, Nentwig GH. Immediate versus delayed functional loading of implants in the posterior mandible: a 2-year prospective clinical study of 12 consecutive cases. Int J Periodontics Restorative Dent. 2006; 26:459–469. PMID: 17073356.
15. Becktor JP, Isaksson S, Sennerby L. Survival analysis of endosseous implants in grafted and nongrafted edentulous maxillae. Int J Oral Maxillofac Implants. 2004; 19:107–115. PMID: 14982363.
16. Shumaker ND, Metcalf BT, Toscano NT, Holtzclaw DJ. Periodontal and periimplant maintenance: a critical factor in long-term treatment success. Compend Contin Educ Dent. 2009; 30:388–390. 392394 passimquiz 407, 418. PMID: 19757733.
17. Cavallaro JS Jr. Implant survival and radiographic analysis of proximal bone levels surrounding a contemporary dental implant. Implant Dent. 2011; 20:146–156. PMID: 21448024.

18. Stanford CM, Wagner W, Baena RRY, Norton M, McGlumphy E, Schmidt J. Evaluation of the effectiveness of dental implant therapy in a practice-based network (FOCUS). Int J Oral Maxillofac Implants. 2010; 25:367–373. PMID: 20369097.
19. Stafford GL. Are the outcomes of immediate and early single tooth implants comparable to conventionally placed implants? Evid Based Dent. 2009; 10:77–78. PMID: 19820741.

20. Kook MS, Park HJ, Kim SG, Kim YK, Cho YS, Choi GL, et al. A retrospective multicenter clinical study of installed US II / SS II implants after maxillary sinus floor elevation. J Korean Assoc Oral Maxillofac Surg. 2008; 34:341–349.
21. Jeong MA, Kim SG, Kim YK, Oh HK, Cho YS, Kim WC, et al. A multicenter prospective study in type IV bone of a single type of implant. Implant Dent. 2012; 21:330–334. PMID: 22814559.

22. Park JH, Lim YJ, Kim MJ, Kwon HB. The effect of various thread designs on the initial stability of taper implants. J Adv Prosthodont. 2009; 1:19–25. PMID: 21165250.

23. Alves CC, Neves M. Tapered implants: from indications to advantages. Int J Periodontics Restorative Dent. 2009; 29:161–167. PMID: 19408478.
24. Song DW, Lee DW, Kim CK, Park KH, Moon IS. Comparative analysis of peri-implant marginal bone loss based on microthread location: a 1-year prospective study after loading. J Periodontol. 2009; 80:1937–1944. PMID: 19961377.

25. Bryant SR. The effects of age, jaw site, and bone condition on oral implant outcomes. Int J Prosthodont. 1998; 11:470–490. PMID: 9922739.
26. Smith RA, Berger R, Dodson TB. Risk factors associated with dental implants in healthy and medically compromised patients. Int J Oral Maxillofac Implants. 1992; 7:367–372. PMID: 1289263.
27. Heberer S, Hildebrand D, Nelson K. Survival rate and potential influential factors for two transitional implant systems in edentulous patients: a prospective clinical study. J Oral Rehabil. 2011; 38:447–453. PMID: 21070328.

28. Renouard F, Nisand D. Impact of implant length and diameter on survival rates. Clin Oral Implants Res. 2006; 17(Suppl 2):35–51. PMID: 16968380.

29. Woo VV, Chuang SK, Daher S, Muftu A, Dodson TB. Dentoalveolar reconstructive procedures as a risk factor for implant failure. J Oral Maxillofac Surg. 2004; 62:773–780. PMID: 15218553.

30. Sbordone L, Toti P, Menchini-Fabris G, Sbordone C, Guidetti F. Implant survival in maxillary and mandibular osseous onlay grafts and native bone: a 3-year clinical and computerized tomographic follow-up. Int J Oral Maxillofac Implants. 2009; 24:695–703. PMID: 19885411.
31. Schliephake H, Neukam FW, Wichmann M. Survival analysis of endosseous implants in bone grafts used for the treatment of severe alveolar ridge atrophy. J Oral Maxillofac Surg. 1997; 55:1227–1233. PMID: 9371112.

32. Ivanoff CJ, Gröndahl K, Sennerby L, Bergström C, Lekholm U. Influence of variations in implant diameters: a 3- to 5-year retrospective clinical report. Int J Oral Maxillofac Implants. 1999; 14:173–180. PMID: 10212533.
33. Aparicio C, Rangert B, Sennerby L. Immediate/early loading of dental implants: a report from the Sociedad Espaola de Implantes World Congress consensus meeting in Barcelona, Spain, 2002. Clin Implant Dent Relat Res. 2003; 5:57–60. PMID: 12831730.
34. Cochran DL, Morton D, Weber HP. Consensus statements and recommended clinical procedures regarding loading protocols for endosseous dental implants. Int J Oral Maxillofac Implants. 2004; 19(Suppl):109–113. PMID: 15635951.
35. Weber HP, Morton D, Gallucci GO, Roccuzzo M, Cordaro L, Grutter L. Consensus statements and recommended clinical procedures regarding loading protocols. Int J Oral Maxillofac Implants. 2009; 24(Suppl):180–183. PMID: 19885445.
36. Chuang SK, Cai T, Douglass CW, Wei LJ, Dodson TB. Frailty approach for the analysis of clustered failure time observations in dental research. J Dent Res. 2005; 84:54–58. PMID: 15615876.

37. Weyant RJ, Burt BA. An assessment of survival rates and within-patient clustering of failures for endosseous oral implants. J Dent Res. 1993; 72:2–8. PMID: 8380286.

Fig. 3
Comparison of the survival function curves between subgroups. SS II, US II, and GS II are Osstem implants (Osstem Implant Co., Ltd.).
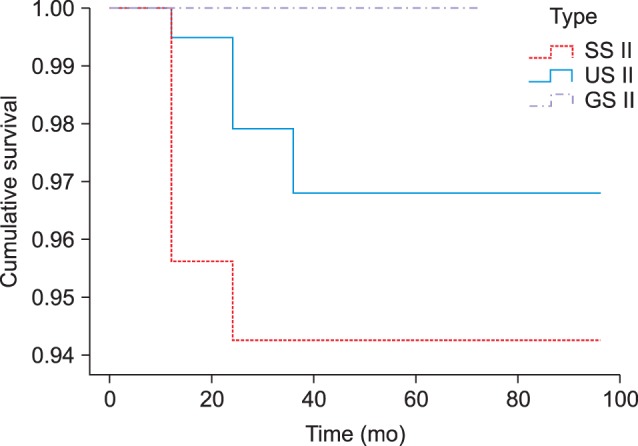
Table 7
Cases of implant failure
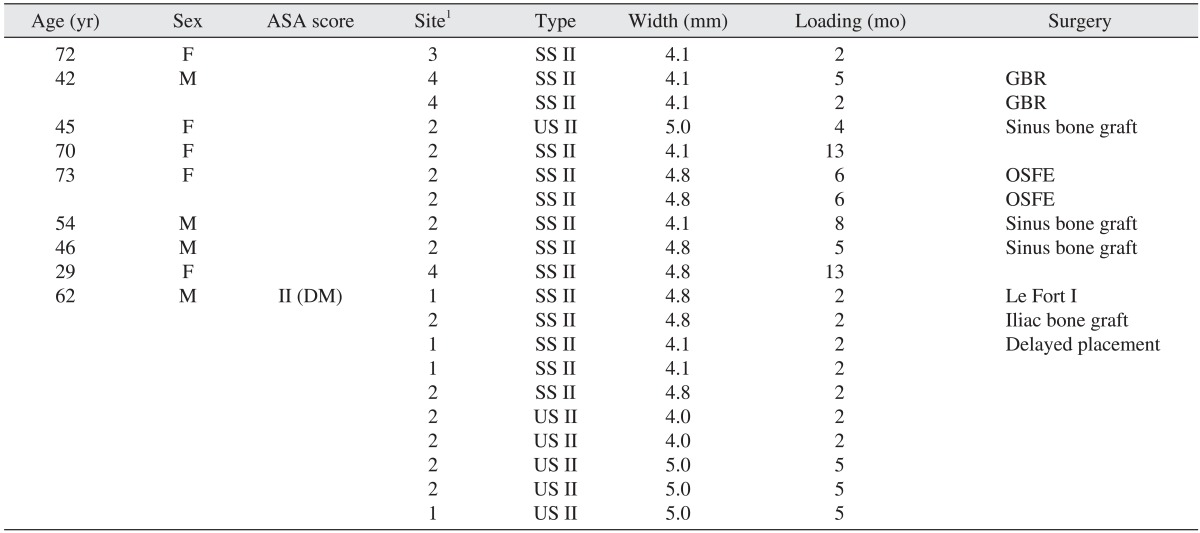
(ASA: American Society of Anesthesiologists, F: female, M: male, DM: diabetes mellitus, GBR: guided bone regeneration, OSFE: osteotome sinus floor elevation)
1Site: 1 (anterior maxilla), 2 (posterior maxilla), 3 (anterior mandible), 4 (posterior mandible).
SS II, US II: Osstem implants (Osstem Implant Co., Ltd.).




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download