Abstract
Cavernous sinus thrombosis not only presents with constitutional symptoms including fever, pain and swelling but also with specific findings such as proptosis, chemosis, periorbital swelling, and cranial nerve palsies. It is known to occur secondary to the spread of paranasal sinus infections in the nose, ethmoidal and sphenoidal sinuses. However, paranasal sinus infection of dental origin is rare. The following is a case of cavernous sinus thrombosis due to the spread of an abscess in the buccal and pterygomandibular spaces via buccal mucosal laceration.
Cavernous sinus thrombosis (CST) was first reported by Bright et al. in 18311. CST is an infectious disease that is potentially fatal if it is not treated in its early stage. Before antibiotics were developed, the mortality rate of CST was almost 100%. Even given medical advances, the mortality rate is still approximately 20%2. Furthermore, despite treatment, complications of CST such as cranial nerve dysfunction may remain.
Dental infections are not common sources of CST. There are very few reports of CST related to periodontal disease or to tooth extraction3. The authors present a case in which misdiagnosis and inappropriate treatment of a buccal cheek laceration due to an ill-fitting denture resulted in a buccal space abscess that progressed into CST.
A 65-year-old man with no past medical history presented to the emergency room with facial swelling and fever. Two weeks earlier, he began having trismus and pain on the mandibular left side. He saw a dentist who diagnosed temporomandibular joint arthralgia and myofacial pain. The patient was prescribed prednisolone, cyclobenzaprine, steroids and muscle relaxants and was advised to frequently apply moist hot packs. The patient's symptoms persisted and he developed spiking fevers with delirium.
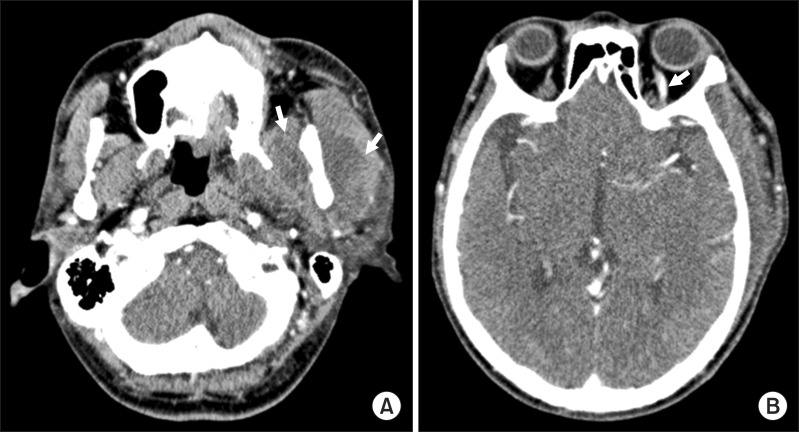
In the emergency room, his vital signs included blood pressure 151/82 mmHg, pulse 95 beats per minute, temperature 39.3℃, and respiratory rate 12/min. A complete blood count revealed a white blood cell (WBC) count of 19,110/µL with 92.8% neutrophils, a erythrocyte sedimentation rate (ESR) of 89 mm/h and C-reactive protein (CRP) of 279.2 mg/L. Computed tomography (CT) showed an abscess in the buccal and pterygomandibular space spreading into the temporal space.(Fig. 1) The patient's mental status was assessed with questions about his personal information. Although he correctly answered every question, his pronunciation was unclear. He had three teeth on the right mandibular side, and the left buccal cheek was swollen with a 1 cm laceration. The origin of the abscess was not clear. However, because the patient showed clinical signs of infection associated with buccal, pterygomandibular, and temporal space abscesses, he was admitted to the hospital. Empirical antibiotic treatment was initiated with flomoxef 2 g/day, metronidazole 1.5 g/day, and isepamicin sulfate 400 mg/day.
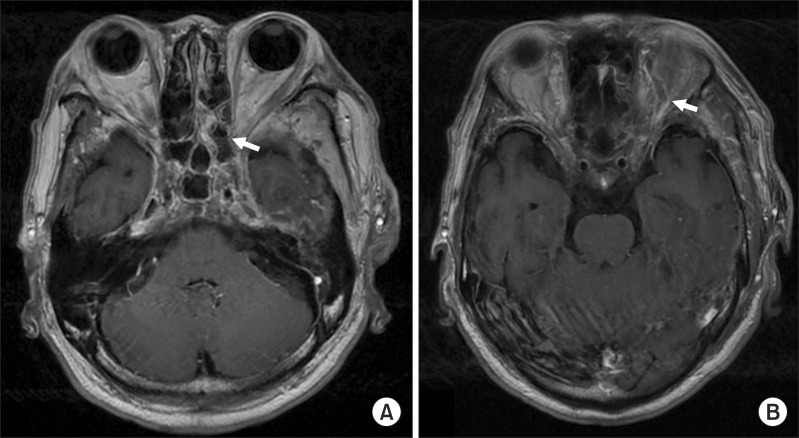
On the day of hospitalization, he also had yellow pus extruding from the left external auditory canal. An ENT doctor confirmed a left eardrum perforation. Ofloxacin otic solution was topically applied to the external auditory canal and the occlusive dressing was changed daily. On the second day of hospitalization, the patient complained of hyperemia, decreased visual acuity and numb extremities. A spinal tap revealed cerebrospinal fluid turbidity suggestive of central nervous system (CNS) infection. Blood cultures were positive for staphylococcus. As per recommendations from internal medicine, and neurology, the antibiotic regimen was changed to ceftazidime 4 g/day and vancomycin 2.7 g/day to cover the CNS infection and potential methicillin-resistance Staphylococcus aureus (MRSA). On the third day of hospitalization, an oral and maxillofacial surgeon performed intraoral incision and drainage of the buccal and pterygomandibular spaces. A large amount of pus was drained. The patient was transferred to the infectious disease division of internal medicine. Results of a magnetic resonance image (MRI) included a dilated superior ophthalmic vein, bulging cavernous sinus contour, and septic thrombi in the cavernous sinus. These findings are reasonable diagnostic signs of CST.(Fig. 2)
Antimicrobial sensitivity testing identified methicillin-sensitive Staphylococcus aureus (MSSA) and therefore the antibiotic regimen was changed to nafcillin 12 g/day. The patient's symptoms improved gradually. His buccal swelling decreased, he recovered the ability to open his mouth, and his visual acuity and extremity sensation improved. WBC, ESR, and CRP counts also improved.(Table 1) With reduced swelling, we discovered an ill-fitting denture. The denture's occlusal margin matched the initial buccal cheek laceration. The patient was stable and healthy on discharge, 23 days after his hospitalization.
CST is a rare condition in which blood clots form within the cavernous sinus, a large collection of thin-walled veins. CST is either an aseptic or septic condition. Aseptic CST usually results from surgery or trauma. Septic CST may be caused by sinusitis, otitis, odontogenic infection, or facial cellulitis. The most frequent cause of septic CST is sinusitis in the sphenoid or ethmoidal sinuses. Dental infections account for <10% of septic CST cases, and most of these cases are related to maxillary infection4. Eagleton5 proposed the following criteria for CST diagnosis: a known site of infection; blood stream infection (sepsis); early obstructive signs (such as retinal vein fullness, proptosis, exophthalmus, and collateral venous circulation); ocular nerve paralysis (via lesions of cranial nerves III, IV, V, and IV); surrounding soft tissue abscesses (of the orbit, occiput, neck or nasopharynx); and symptoms of a complicated disease (such as headache, papilledema, and meningeal signs).
Staphylococcus accounts for approximately 70% of CST, and streptococcal species account for 20%. Other potential pathogens include pneumococcus, bacteroides, fusobacterium, proteus, haemophilus, pseudomonas, and corynebacterium6. Venous flow is bidirectional in the cerebral vein, emissary vein, and dural sinus because these vessels do not have valves. Therefore, bacteria or thrombi from another facial region can spread to the cavernous sinus via the facial vein or pterygoid plexus7.
The clinical symptoms of CST vary depending upon the anatomical structures involved. The most common symptoms are fever, proptosis, chemosis, and external ophthalmoplegia. Ophthalmoplegia is extraocular muscle weakness that results (in CST) when cranial nerves III, IV, and/or VI are damaged due to their passage through the cavernous sinus. Other symptoms of lethargy, headache, periorbital swelling, papilledema, and venous engorgement occur in 50%-80% of patients. Decreased visual acuity, sluggish/dilated pupil, periorbital sensory loss, and decreased corneal reflex are less frequent symptoms that occur in <50% of patients. These symptoms occur when cranial nerve III or V is involved8. Early symptoms of orbital cellulitis are similar to those of CST and include periorbital swelling, proptosis, chemosis, fever and impaired vision. CST is included in the differential diagnosis when the above symptoms present bilaterally or when there is sensory loss in the periocular area, papilledema, and pupillary dilation9.
CT and MRI are the diagnostic modalities of choice for CST. In CST, coronal CT reveals that the lateral wall of the cavernous sinus is flat or convex instead of its normal concave shape. Additionally, irregular filling defects due to thrombi, and superior ophthalmic vein dilation are often observed. MRI is useful when the CT images are not clear or when the infection has spread to surrounding tissues including the brain and pituitary gland10,11. In the past, cerebral angiography and orbital venography were used. These imaging techniques are no longer recommended because they increase the risks of the infection spreading and thrombosis12.
Optimal therapy for CST includes the concomitant administration of nafcillin, metronidazole, and ceftriaxone/cefotaxime. If a MRSA infection is suspected, vancomycin may be administered. Surgical interventions are indicated when the primary site of infection is sinusitis, or a dental infection. In the case of a serious infection, one may consider direct surgical drainage of the cavernous sinus; however, this approach is difficult and complications are likely to arise7.
Corticosteroid therapy must be considered if adrenal insufficiency occurs due to cranial nerve dysfunction or pituitary necrosis13. Some studies have reported that anticoagulating with heparin, for example, within seven days after CST reduces the mortality rate14. However, anticoagulation increases the risk of intracranial and/or systemic hemorrhage, and therefore should be used with caution15.
In this case, it was difficult to confirm the source of infection because the patient's buccal cheek swelling and trismus prevented adequate intraoral examination. Furthermore, there were only three teeth on the side contralateral to the infection. In general, there were no significant intraoral findings. The only suspicious finding was a small laceration on left buccal mucosa. Generally, odontogenic infections arise from the teeth themselves or from periodontal tissue. Therefore, it was difficult to confirm that this severe infection originated from a small laceration in a patient with no medical history. Another dentist who examined the patient two weeks before his presentation had misdiagnosed him with temporomandibular joint symptoms and myofascial pain. Administration of moist hot packs and prednisolone may have actually facilitated the infection's progression.
The patient was treated with incision and drainage, and antibiotics. CST treatment must include proper management of the primary infection site and empiric antibiotics considering the possible pathogens. CST presents with inconsistent symptoms, and therefore it is essential to collaborate with various departments including neurology, ophthalmology, otorhinolaryngology, and internal medicine.
CST can be fatal when a thrombus forms in the cavernous sinus. It presents with proptosis, chemosis, periorbital swelling and other typical signs of infection including as fever, pain and swelling. The development of antibiotics has decreased the mortality of CST. However, the mortality rate is still relatively high at 14%-30%. This disease requires early diagnosis and proper treatment. Even after treatment, cranial nerve dysfunction may remain and intra-cavernous aneurysm may also occur. Therefore, long-term patient follow-up is imperative. Clinicians in oral and maxillofacial surgery should be aware of CST and should consider it in patients with facial swelling.
References
1. Tempea V, Gorun G. Cavernous sinus thrombosis. AMA Arch Otolaryngol. 1959; 69:220–223. PMID: 13616816.

2. Yarington CT Jr. Cavernous sinus thrombosis revisited. Proc R Soc Med. 1977; 70:456–459. PMID: 331338.
3. Colbert S, Cameron M, Williams J. Septic thrombosis of the cavernous sinus and dental infection. Br J Oral Maxillofac Surg. 2011; 49:e25–e26. PMID: 20691517.

4. Yarington CT Jr. The prognosis and treatment of cavernous sinus thrombosis. Review of 878 cases in the literature. Ann Otol Rhinol Laryngol. 1961; 70:263–267. PMID: 13787240.
5. Eagleton WP. Cavernous sinus thrombophlebitis and allied septic and traumatic lesions of the basal venous sinuses: a clinical study of blood stream infection. New York: Macmillan;1926.
6. Desa V, Green R. Cavernous sinus thrombosis: current therapy. J Oral Maxillofac Surg. 2012; 70:2085–2091. PMID: 22326173.

7. DiNubile MJ. Septic thrombosis of the cavernous sinuses. Arch Neurol. 1988; 45:567–572. PMID: 3282499.

8. Southwick FS, Richardson EP Jr, Swartz MN. Septic thrombosis of the dural venous sinuses. Medicine (Baltimore). 1986; 65:82–106. PMID: 3512953.

9. Price CD, Hameroff SB, Richards RD. Cavernous sinus thrombosis and orbital cellulitis. South Med J. 1971; 64:1243–1247. PMID: 5097800.

10. Schuknecht B, Simmen D, Yüksel C, Valavanis A. Tributary venosinus occlusion and septic cavernous sinus thrombosis: CT and MR findings. AJNR Am J Neuroradiol. 1998; 19:617–626. PMID: 9576645.
11. Ben-Uri R, Palma L, Kaveh Z. Septic thrombosis of the cavernous sinus: diagnosis with the aid of computed tomography. Clin Radiol. 1989; 40:520–522. PMID: 2791467.
12. Berge J, Louail C, Caillé JM. Cavernous sinus thrombosis diagnostic approach. J Neuroradiol. 1994; 21:101–117. PMID: 8014656.
13. Solomon OD, Moses L, Volk M. Steroid therapy in cavernous sinus thrombosis. Am J Ophthalmol. 1962; 54:1122–1124. PMID: 13978082.
14. Levine SR, Twyman RE, Gilman S. The role of anticoagulation in cavernous sinus thrombosis. Neurology. 1988; 38:517–522. PMID: 3281056.

15. Endo S, Ohtsuji T, Fukuda O, Oka N, Takaku A. A case of septic cavernous sinus thrombosis with sequential dynamic angiographic changes. A case report. Surg Neurol. 1989; 32:59–63. PMID: 2734690.

Fig. 1
Computed tomography scans with contrast. A. Large lobulated abscess pocket formation in the left buccal and pterygomandibular space (arrows). B. Venous dilation of the left superior ophthamic vein (arrow).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download