Abstract
Objectives
The purpose of this preliminary study is to evaluate the effectiveness of a customized, three-dimensional, preformed titanium mesh as a barrier membrane for peri-implant alveolar bone regeneration.
Materials and Methods
Ten patients were recruited for this study. At the time of implant placement, all patients had fenestration or a dehiscence defect around the implant fixture. A mixture of particulate intraoral autologous bone and freeze-dried bone allograft was applied to the defect in a 1 : 1 volume ratio and covered by the preformed titanium mesh. A core biopsy specimen was taken from the regenerated bone four months postoperatively. Patients were followed for 12 months after the definitive prosthesis was placed.
Results
Satisfactory bone regeneration with limited fibrous tissue was detected beneath the preformed titanium mesh. Histologic findings revealed that newly formed bones were well-incorporated into the allografts and connective tissue. New growth was composed of approximately 80% vital bone, 5% fibrous marrow tissue, and 15% remaining allograft. All implants were functional without any significant complications.
Conclusion
The use of preformed titanium mesh may support bone regeneration by maintaining space for new bone growth through its macro-pores. This preliminary study presents the efficacy of a preformed titanium mesh as a ready-to-use barrier membrane around peri-implant alveolar bone defect. This preformed mesh is also convenient to apply and to remove.
Go to : 
Dental implantation is an effective therapeutic option to replace missing teeth in modern dentistry. In order for an implant to successfully sustain functional loads, there must be sufficient alveolar bone around the implant. Implants must be at least 5 mm wide and 7-10 mm in length1. After implantation, dehiscence or fenestration defects frequently occur because of insufficient residual bone volume. Bone augmentation should be performed prior to and/or simultaneously with implant placement when the preoperative examination shows a lack of alveolar bone volume at the implant placement site. Alveolar bone defects may be treated with various bone regeneration techniques including block bone graft, guided bone regeneration (GBR), ridge splitting, and distraction osteogenesis2,3,4,5. GBR is one of the most predictable methods, which mostly uses a barrier membrane to separate the grafted defect and the surrounding connective tissue for successful bone regeneration3. Barrier membranes are either resorbable or non-resorbable membranes. Two major types of non-resorbable barrier membranes are expanded polytetra-fluoroethylene (e-PTFE) and titanium mesh6. Despite many advantages of GBR, it is difficult to maintain suitable space for ideal bone regeneration because unless there is a hard structure to support the space, soft tissue collapses into it.
Titanium mesh has been widely used for oral and maxillofacial defect reconstruction7. It is biocompatible and rigid enough to maintain the grafted space6. It has been applied to various defects indicated for GBR, including sinus augmentation sites8,9. Several studies have used GBR procedures with titanium mesh and various bone graft materials including autologous bone, bovine bone mineral, alloplastic materials, and their combinations8,10,11,12,13,14. However, studies that have used titanium mesh and allogeneic graft, especially freeze-dried bone allograft (FDBA), are rare. Few of these studies present histologic evidence of bone maturation on the grafted site with titanium mesh and FDBA.
One complication related to titanium mesh is the high risk of exposure following repeated mucosal irritation. Although titanium mesh exposure does not always lead to bone augmentation failure in a clinical setting8,13, the bone quality of GBR would be adversely affected15. Before its application, titanium mesh should be cut, bent, and trimmed. Any prominent, sharp edges resulting from these manipulations may cause membrane exposure. Recently, preformed titanium mesh with round and blunt edges were developed to prevent mucosal irritation.
We used a customized, three-dimensional, preformed titaniumn mesh (SMARTbuilder; Osstem, Busan, Korea) to reconstruct the peri-implant defects occurring immediately after implant placement. Clinical and histologic investigations were used to evaluate the preformed titanium mesh's efficacy as a barrier membrane against a mixture of autologous and FDBA in localized alveolar bone regeneration.
Go to : 
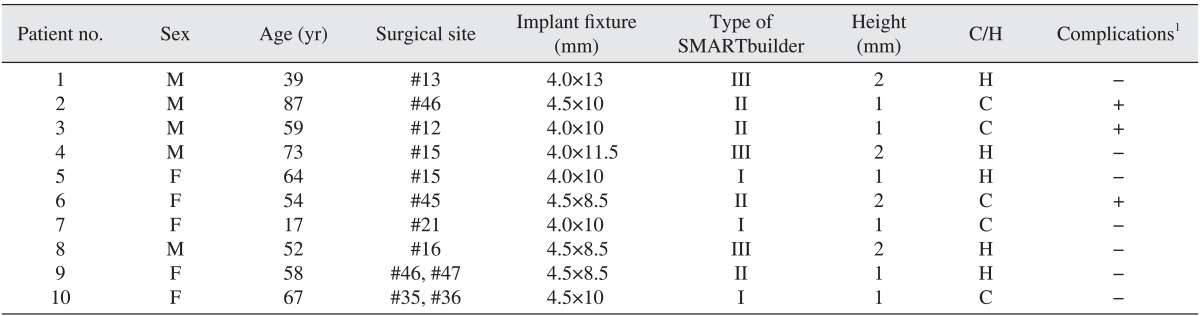
A total of 10 patients (5 male and 5 female, aged 17-87 years) who were partially edentulous participated in this study between August 2012 and June 2014 at Hanyang University Hospital. Preoperative clinical and radiologic evaluations indicated that the GBR procedure should be performed simultaneously with the implant placement in all patients. Guidelines of the Helsinki Declaration were followed during all treatment phases. This study was approved by the Institutional Review Board of Hanyang University Hospital (HYH IRB 2012-06-014).
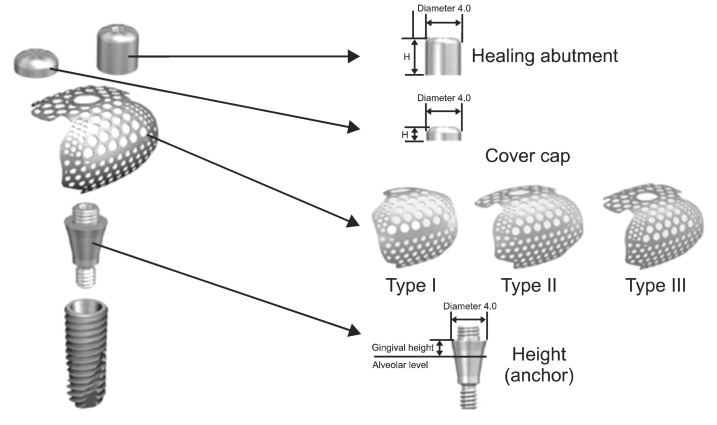
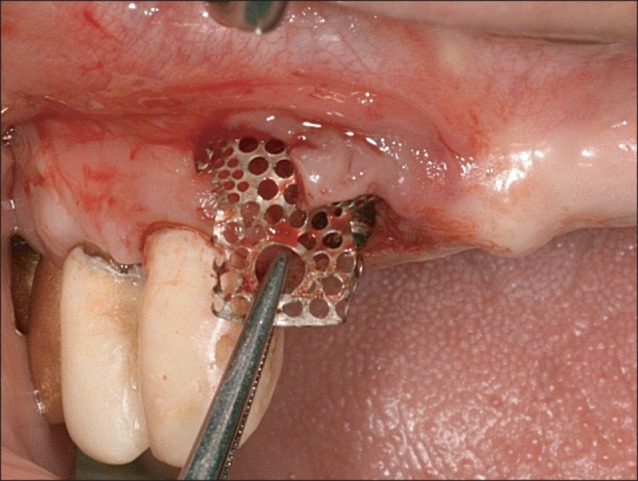
Implant were placed under local anesthesia using a trapezoidal full-thickness flap with two vertical releasing incisions. A total of 12 implants (TS III CA; Osstem) in 10 patients were inserted by the standard procedure. In all cases, the implant threads were partially exposed and the GBR procedure was performed for these peri-implant defects. The preformed titanium mesh was selected according to the defect size and its configurations.(Fig. 1) The height (or anchor) was connected to the implant fixture. Then, the preformed titanium mesh was located on that height and immobilized by cover cap for a staged approach or by healing abutment for transmucosal GBR. The height, cover cap, and healing abutment were specifically designed for the preformed titanium mesh.(Fig. 1)
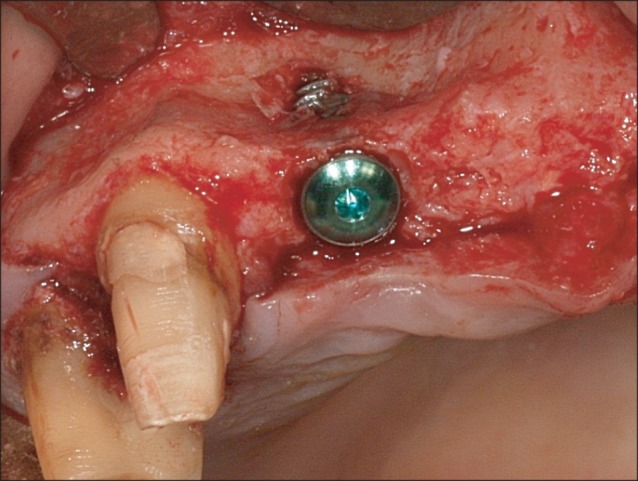
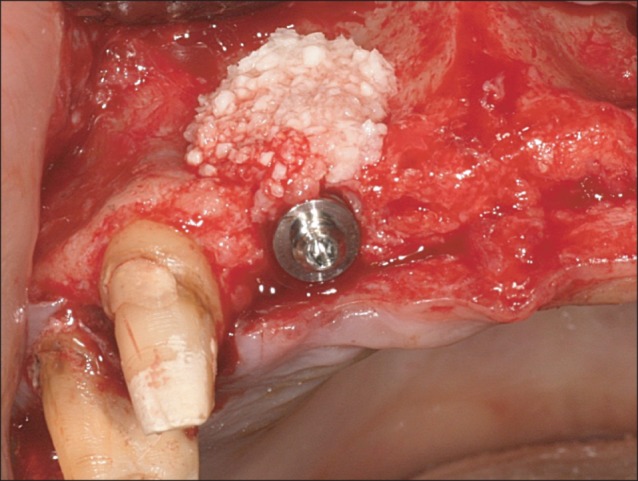
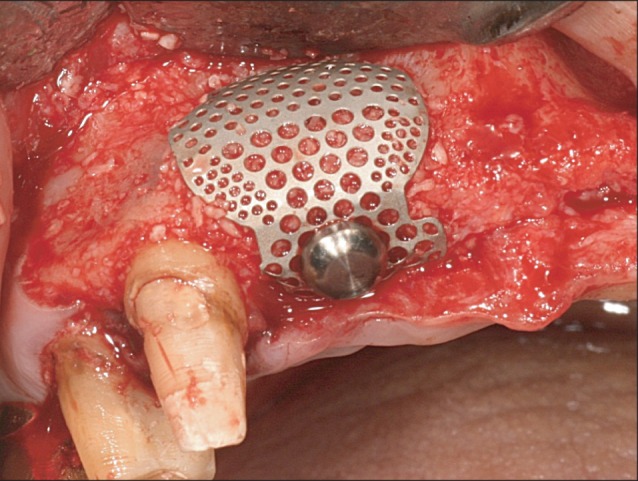
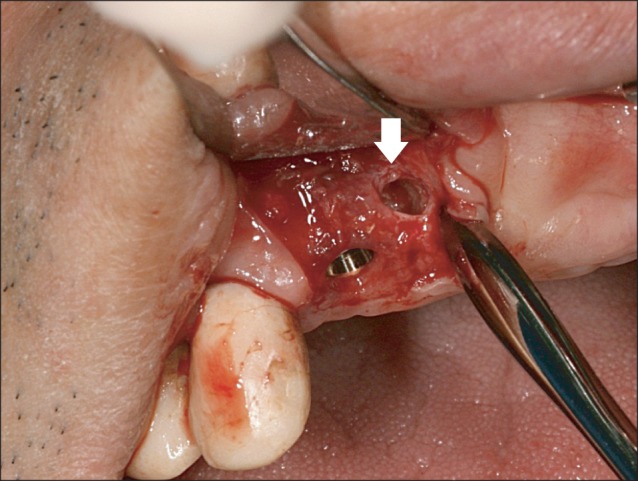
All 10 patients demonstrated alveolar bone fenestration or dehiscence after implant placement.(Fig. 2) An Autobone Collector (Osstem) was used to harvest autologous bone chips were either from the mandibular ramus or from an area adjacent to the surgical site. The bone chips were mixed with FDBA (Sure-Oss; HansBiomed, Seoul, Korea) in 1 : 1 volume ratio. A mixture of the graft materials was placed on the dehiscence or fenestration defect (Fig. 3) and covered with the preformed titanium mesh.(Fig. 4) Primary flap closure was accomplished without tension. Antibiotics and analgesics were administered three times daily for ten days in addition to 0.12% chlorhexidine mouth rinse. The sutures were removed two weeks after surgery, and the patients were followed every four weeks after surgery for wound assessment.
After approximately four months of the healing period, re-entry surgery was performed to uncover the implant under the minimum flap reflection. The preformed titanium mesh was removed and the regenerated bone was examined carefully.(Fig. 5) A trephine drill with a 2.2 mm internal diameter was used to obtain the core biopsy from the regenerated site.(Fig. 6) Next, a routine healing abutment and sutures were placed. Two months after the re-entry surgery, both the osseointegration and the integrity of the peri-implant tissue were evaluated. Patients were referred to the prosthodontic department. The complications and implant success rate were investigated for twelve months after the definite prosthesis was placed.
The specimen obtained at re-entry was immediately fixed in 10% buffered formalin, dehydrated in a series of ethanol baths, and embedded in methyl methacrylate for histologic evaluation. The polymerized blocks were sectioned with a diamond saw microtome, micro-grinded and polished using sandpaper. Final sections were approximately 40 µm thick. The sections were stained with H&E. Histomorphometric analysis was performed with light microscope. The areas of vital bone, residual graft materials, and soft tissue were grossly measured and analyzed.
Go to : 
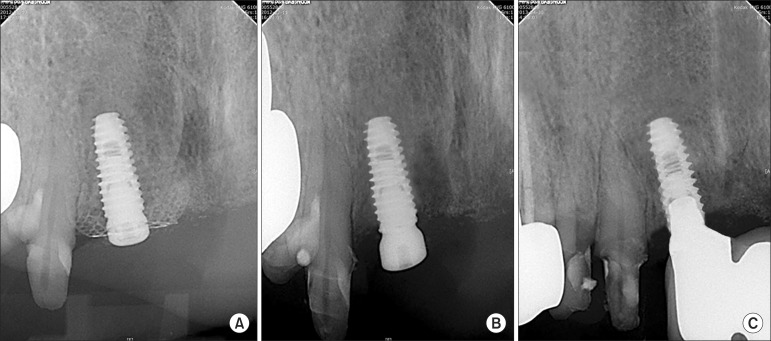
Patient characteristics and their surgical records are summarized in Table 1. Newly regenerated bone at the augmented sites was confirmed by re-entry at four months after the initial surgery. After removing the preformed titanium mesh, a thin connective tissue layer called "pseudoperiosteum" was observed, but not debrided16. Underneath the pseudoperiosteum, FDBA particles were found to be embedded and incorporated in the regenerated bone. Postsurgical complications were observed in three of the 10 patients (30%). In two of these patients, the titanium mesh became exposed and there was small flap dehiscence on the cover cap in one patient. During the healing period, the exposed titanium meshes and cover caps were frequently irrigated with 0.12% chlorhexidine until fibrous tissue formed beneath the titanium mesh and graft materials were no long lost into the oral cavity. No signs of infection or suppuration were identified in the soft tissue around the exposed area. In all cases, the quality and quantity of the regenerated bone was clinically acceptable; however, at the exposed site, there was some resorption of graft materials. All implants were functional without significant complications. Radiologic evaluation at mesh insertion, removal and 12 months after definitive prostheses were placed showed stable marginal bone in all implants.(Fig. 7)
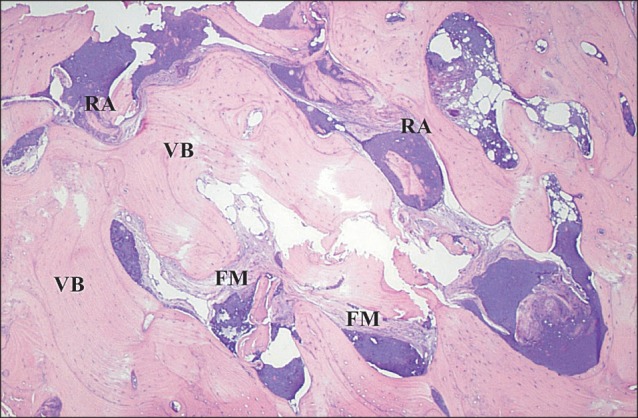
Histologic findings of the core specimen from patient #2 revealed that the allograft particles are well-incorporated and surrounded by newly formed vital bone and connective tissue.(Fig. 8) Vital bone surrounded ~80% of the allograft, while fibrous marrow tissue surrounded 5% of the graft, and inflammatory cell infiltrates made up the remaining 15%.
Go to : 
Adequate bone volume is a prerequisite for the long-term success of endosseous implants. Insufficient bone must be reconstructed with various hard tissue augmentation procedures2,3,4. In order for augmentation procedures to be successful, it is imperative to stabilize the graft during healing, support the osteogenic potential of the graft materials, and close the primary soft tissue.
GBR is often performed during implant placement to correct the peri-implant alveolar defects, and is considered a favorable procedure. The GBR technique uses a barrier membrane to prevent soft tissue cells from entering the grafted space, maintaining the space for successful bone regeneration. Actually, the most important ability of GBR is its ability to maintain space under the membrane. This preliminary study focused on a titanium mesh, which is one of the most reliable barrier membranes in ridge augmentation. The titanium mesh was first proposed for alveolar ridge reconstruction in 1969 by Boyne7. It is remarkably biocompatible and resists corrosion and thermal effects. Based on these qualities, titanium mesh has been useful in the reconstruction and augmentation of oral and maxillofacial defects. Another advantage of the titanium mesh is its inherent rigidity that maintains the space needed to allow new bone growth. Membrane stability is necessary to maintain graft volume during wound healing17. The titanium mesh's rigidity was ideal for vertical and horizontal augmentation14. Several studies have demonstrated that titanium mesh supports the grafted space and prevents soft tissue collapse better than does e-PTFE6,14. In contrast, although some cross-linked collagen membranes are also rigid resorbable barrier membranes, they only protect the grafted space at the beginning of regeneration and gradually lose their rigidity6.
One of the prerequisites of successful GBR is a barrier mem brane that restricts epithelial cell migration. Unlike other barrier membranes, titanium mesh has a macro-pore structure. This distinguishing feature is thought to induce the biological activity of the graft materials6. The macro-pores allow sufficient blood flow and diffusion of nutrients and oxygen that consequently enhance the regeneration of the bone and soft tissue. As the membrane pore size of increases, so does the regenerative capacity. Despite concern that epithelial cells and bacteria could infiltrate through large pores, space maintenance was found to actually play a greater role in bone regeneration than does cell occlusiveness18. When blood clot stabilization is achieved with the titanium mesh, epithelial tissue may be excluded during GBR13. In addition, the titanium mesh with large pores demonstrates more osseous formation and less soft tissue attachment than does a titanium mesh with small pores12. We found that easily removable titanium meshes tend to have less adhesive fibrous tissue formation. The titanium meshes used in this study have two different pore sizes that permit the migration of bone-forming cells, but block the grafted materials from leaking.
A major limitation of the titanium mesh is the fairly high rate of mesh exposure through the tissue. Corinaldesi et al.8 reported a exposure rate of 14.8% after particulated bone augmentation in the mandible. Louis et al.14 reported 52% exposure of the maxilla and mandible with a range of 43%-55%. Three of the 10 patients (30%) in this study experienced titanium mesh exposure during the healing period (with one in the maxilla and two in the mandible). However, even in cases of membrane exposure, there was satisfactory bone regeneration and successful implant survival. The most common cause of mesh exposure is continuous mucosal irritation by the titanium mesh. Sharp edges produced by improper mesh adjustment may increase the risk of exposure. Using a preformed titanium mesh is ideal because there is no need to cut or bend the mesh. Having a preformed mesh not only shortens the duration of surgery, but also reduces the risk of mesh exposure. In our study, there were no titanium mesh edge exposures because the preformed titanium meshes had optimal round and blunt edges. Ciocca et al.10 also prepared a customized titanium mesh using computer-aided design and computer-aided manufacturing (CAD/CAM) technology to augment the atrophic alveolar ridge and demonstrated satisfactory bone regeneration.
Few studies have reported the histological results of new bone formation with titanium mesh use in humans. Proussaefs and Lozada19 demonstrated ~36% new bone formation after 8 months in a histologic study of cases augmented by mixed grafts of autologous and inorganic bovine bone (in a 1 : 1 volume ratio). Using the mesh with either a combination graft (of 70% autologous bone and 30% bovine bone matrix) or a graft of autologous bone alone produces similar amounts of total bone volume 8-9 months after grafting11. In accordance with previous studies using allogeneic grafts20,21, preliminary histologic analysis in our study demonstrated a satisfactory amount of new bone formation with limited allograft particle embedding. The newly formed bone was surrounded and focally merged with FDBA particles. It is thought that FDBA scaffolding connects with marrow trabeculae, providing favorable osteoconductivity for new bone formation. Moreover, marginal bone levels around the implant remained stable for 12 months after the final prostheses were placed. There were no failures of osseointegration or GBR in this experiment.
Go to : 
The customized, three-dimensional, and preformed titanium mesh used in combination with autologous bone and FDBA induced successful bone regeneration in peri-implant defects occurring after implant placement. Even in the cases of titanium mesh exposure, this preformed titanium mesh produced reliable outcomes as a barrier membrane. This preliminary study shows histologic evidence of satisfactory vital bone formation in the augmented area. Preformed titanium mesh supports the grafted space for new bone formation, makes application and removal convenient, and minimizes the risk of mesh exposure in the reconstruction of peri-implant alveolar bone defects.
Go to : 
References
1. Brånemark PI, Zarb GA, Albrektsson T. Tissue-integrated prostheses: osseointegration in clinical dentistry. Chicago: Quintessence;1985. p. 199–209.
2. Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997; 12:767–776. PMID: 9425757.
3. Hämmerle CH, Karring T. Guided bone regeneration at oral implant sites. Periodontol 2000. 1998; 17:151–175. PMID: 10337322.

4. Simion M, Baldoni M, Zaffe D. Jawbone enlargement using immediate implant placement associated with a split-crest technique and guided tissue regeneration. Int J Periodontics Restorative Dent. 1992; 12:462–473. PMID: 1298734.
5. Oda T, Sawaki Y, Ueda M. Experimental alveolar ridge augmentation by distraction osteogenesis using a simple device that permits secondary implant placement. Int J Oral Maxillofac Implants. 2000; 15:95–102. PMID: 10697943.
6. Rakhmatia YD, Ayukawa Y, Furuhashi A, Koyano K. Current barrier membranes: titanium mesh and other membranes for guided bone regeneration in dental applications. J Prosthodont Res. 2013; 57:3–14. PMID: 23347794.

7. Boyne PJ. Restoration of osseous defects in maxillofacial casualities. J Am Dent Assoc. 1969; 78:767–776. PMID: 4975262.
8. Corinaldesi G, Pieri F, Sapigni L, Marchetti C. Evaluation of survival and success rates of dental implants placed at the time of or after alveolar ridge augmentation with an autogenous mandibular bone graft and titanium mesh: a 3- to 8-year retrospective study. Int J Oral Maxillofac Implants. 2009; 24:1119–1128. PMID: 20162118.
9. Papadogeorgakis N, Prokopidi ME, Kourtis S. The use of titanium mesh in sinus augmentation. Implant Dent. 2010; 19:109–114. PMID: 20386213.

10. Ciocca L, Fantini M, De Crescenzio F, Corinaldesi G, Scotti R. Direct metal laser sintering (DMLS) of a customized titanium mesh for prosthetically guided bone regeneration of atrophic maxillary arches. Med Biol Eng Comput. 2011; 49:1347–1352. PMID: 21779902.

11. Corinaldesi G, Pieri F, Marchetti C, Fini M, Aldini NN, Giardino R. Histologic and histomorphometric evaluation of alveolar ridge augmentation using bone grafts and titanium micromesh in humans. J Periodontol. 2007; 78:1477–1484. PMID: 17668966.

12. Gutta R, Baker RA, Bartolucci AA, Louis PJ. Barrier membranes used for ridge augmentation: is there an optimal pore size? J Oral Maxillofac Surg. 2009; 67:1218–1225. PMID: 19446207.

13. Her S, Kang T, Fien MJ. Titanium mesh as an alternative to a membrane for ridge augmentation. J Oral Maxillofac Surg. 2012; 70:803–810. PMID: 22285340.

14. Louis PJ, Gutta R, Said-Al-Naief N, Bartolucci AA. Reconstruction of the maxilla and mandible with particulate bone graft and titanium mesh for implant placement. J Oral Maxillofac Surg. 2008; 66:235–245. PMID: 18201602.

15. von Arx T, Hardt N, Wallkamm B. The TIME technique: a new method for localized alveolar ridge augmentation prior to placement of dental implants. Int J Oral Maxillofac Implants. 1996; 11:387–394. PMID: 8752560.
16. Boyne PJ, Cole MD, Stringer D, Shafqat JP. A technique for osseous restoration of deficient edentulous maxillary ridges. J Oral Maxillofac Surg. 1985; 43:87–91. PMID: 3881576.

17. Adeyemo WL, Reuther T, Bloch W, Korkmaz Y, Fischer JH, Zöller JE, et al. Healing of onlay mandibular bone grafts covered with collagen membrane or bovine bone substitutes: a microscopical and immunohistochemical study in the sheep. Int J Oral Maxillofac Surg. 2008; 37:651–659. PMID: 18378427.

18. Lundgren AK, Sennerby L, Lundgren D, Taylor A, Gottlow J, Nyman S. Bone augmentation at titanium implants using autologous bone grafts and a bioresorbable barrier. An experimental study in the rabbit tibia. Clin Oral Implants Res. 1997; 8:82–89. PMID: 9758958.

19. Proussaefs P, Lozada J. Use of titanium mesh for staged localized alveolar ridge augmentation: clinical and histologic-histomorphometric evaluation. J Oral Implantol. 2006; 32:237–247. PMID: 17069168.

20. Feuille F, Knapp CI, Brunsvold MA, Mellonig JT. Clinical and histologic evaluation of bone-replacement grafts in the treatment of localized alveolar ridge defects. Part 1: Mineralized freeze-dried bone allograft. Int J Periodontics Restorative Dent. 2003; 23:29–35. PMID: 12617366.
21. Wang HL, Tsao YP. Histologic evaluation of socket augmentation with mineralized human allograft. Int J Periodontics Restorative Dent. 2008; 28:231–237. PMID: 18605598.
Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download