Abstract
Objectives
The purpose of this study was to investigate the neurogenic differentiation of human dental pulp stem cells (DPSCs), periodontal ligament stem cells (PDLSCs), and stem cells from apical papilla (SCAP).
Materials and Methods
After induction of neurogenic differentiation using commercial differentiation medium, expression levels of neural markers, microtubule-associated protein 2 (MAP2), class III β-tubulin, and glial fibrillary acidic protein (GFAP) were identified using reverse transcriptase polymerase chain reaction (PCR), real-time PCR, and immunocytochemistry.
Results
The induced cells showed neuron-like morphologies, similar to axons, dendrites, and perikaryons, which are composed of neurons in DPSCs, PDLSCs, and SCAP. The mRNA levels of neuronal markers tended to increase in differentiated cells. The expression of MAP2 and β-tubulin III also increased at the protein level in differentiation groups, even though GFAP was not detected via immunocytochemistry.
Conclusion
Human dental stem cells including DPSCs, PDLSCs, and SCAP may have neurogenic differentiation capability in vitro. The presented data support the use of human dental stem cells as a possible alternative source of stem cells for therapeutic utility in the treatment of neurological diseases.
It is well-known that stem cells (human embryonic stem cells, mesenchymal stem cells derived from various human tissue, and induced pluripotent stem cells) have diverse lineage differentiation potential and self-renewal capacity1,2,3,4,5. These cells have been studied extensively for applications in the field of regenerative medicine. Recently, dental pulp, periodontal ligaments, alveolar bone marrow, pulp of human exfoliated deciduous teeth, and apical papilla have all been studied as possible sources of dental stem cells6,7,8,9. Stem cells obtained from dental tissues are promising candidates, because they can be easily obtained from extracted human third molars, which are usually discarded after removal.
We have previously reported that dental pulp stem cells (DPSCs) and periodontal ligament stem cells (PDLSCs) can be differentiated into osteoblasts and adipocytes9,10,11. In addition, stem cells from apical papilla (SCAP) were previously reported to have differentiation capacity for osteogenesis and adipogenesis12. In vivo transplantation results show that these cells have produced different histomorphological findings. This means that the source of cells can influence the transplantation results, which reflect the origin of the adult stem cells sources9,10,11. Notably, dental stem cells can differentiate into neural crest-derived cells, such as neurons and glial cells, because they are obtained from tissue within the neural crest13,14,15. In some reports, dental stem cells show better neural stem cell properties than bone marrow-derived mesenchymal stem cells.16 Although it has been recently reported that transdifferentiation of mesenchymal stem cells to neuroectoderm or epithelium is possible, the neurogenesis of dental stem cells, including DPSCs, PDLSCs, and SCAP, has not yet been fully elucidated.
Therefore, the present study was conducted to confirm the neurogenic differentiation potential of human DPSCs, PDLSCs, and SCAP in vitro using reverse transcriptase polymerase chain reaction (PCR), real-time PCR, and immunocytochemistry.
Human dental stem cells were harvested from extracted human third molars. These impacted third molars were obtained from adults (18 to 27 years old, n=3) who underwent third molar extraction at the Department of Oral and Maxillofacial Surgery at Seoul National University Dental Hospital. All patients gave informed consent for the purpose and procedures of this study, which was approved by the Institutional Review Board at School of Dentistry, Seoul National University (IRB No. S-D20080009). Periodontal ligaments and root apical papilla were gently separated from the root surface, and dental pulp was obtained from the cementoenamel junction cut by an odontomy procedure to expose the pulp chamber. Each tissue was chopped into pieces and digested in 1 mg/mL type I collagenase (BioBasic Inc., Toronto, ON, Canada) and 2.4 mg/mL dispase (Gibco BRL, Grand Island, NY, USA) for 1 hour by shaking at 37℃ in a 5% CO2 incubator to generate a single-cell suspension. The cells were cultured in α-MEM (Gibco BRL) supplemented with 15% fetal bovine serum (Equitech-Bio Inc., Kerrville, TX, USA), 2 mM L-glutamine (Gibco BRL), 100 µM L-ascorbate-2-phosphate (Sigma-Aldrich, St. Louis, MO, USA), and 1% antibiotic-antimycotic (Gibco BRL). The cells were incubated at 37℃ in 95% humidified air and 5% CO2. Beginning after seven to ten days, the medium was replaced every two days, and the cells were sub-cultured at 70% confluence; the colonies of DPSCs, PDLSCs, and SCAP were observed in respective culture dishes.
Culture wells were coated with poly-L-ornithine (100 µg/mL final concentration; Sigma-Aldrich) overnight at 37℃ in a humidified incubator. Wells were washed with distilled water and coated with laminin (4 µg/mL final concentration; Sigma-Aldrich) overnight at 37℃ in an incubator. Coated wells were washed with phosphate-buffered saline (PBS) and then used for neurogenic differentiation culture. For the induction of neural differentiation, at passage two, DPSCs, PDLSCs, and SCAP were sub-cultured in multi-well plates and grown until 80% confluence. Control samples were maintained in proliferation medium, while neurogenic induction samples were changed to neurogenic differentiation medium (Promo-Cell, Heidelberg, Germany). After 24 hours, RNA was isolated from cells in six-well plates to confirm gene expression, and the two-well coated chamber slides were fixed with 10% formalin for immunocytochemistry staining.
Total RNA was isolated from cells using an RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA concentration was calculated using a Nanodrop ND2000 spectrophotometer (Nano-Drop Technologies, Wilmington, DE, USA). The reverse transcription reaction was performed using a SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. RT-PCR was performed using 1 µg of cDNA in a GoTaq Green Master Mix (Promega, Madison, WI, USA) with 10 pmole of each primer. The following is a list of primer sets and annealing temperatures used: microtubule-associated protein 2 (MAP2) (forward, CATGGGTCACAGGGCACCTATTC; reverse, GGTGGAGAAGGAGGCAGATTAGCTG; annealing temperature, 63℃), β-tubulin, class III (forward, GGCCTCTTCTCACAAGTACG; reverse, CCACTCTGACCAAAGATGAAA; 57℃), glial fibrillary acidic protein (GFAP) (forward, CCGACAGCAGGTCCATGTG; reverse, GTTGCTGGACGCCATTGC; 60℃), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (forward, AGCCGCATCTTCTTTGCGTC; reverse, TCATATTTGGCAGGTTTTTCT; 57℃). Additionally, RT-PCR was performed with primers of neurofilamnet-medium chain (NF-M), neuron specific enolase (NSE), S100, and choline acetyltransferase (ChAT).(Supplementary Table 1) PCR products were analyzed by electrophoresis in 1.5% agarose gels (BioBasic Inc.). For PCR quantification, real-time PCR was performed in triplicate using SYBR Green (Applied Biosystems, Foster City, CA, USA) and a Real-Time PCR System 7500 (Applied Biosystems). Thermal cycling conditions included a 5-minute step at 95℃, followed by 95℃ for 10 seconds and 60℃ for 33 seconds. These steps were repeated for 40 cycles with melting curve analysis. Relative quantification of each gene was calculated after normalization to GAPDH by using the 2-ΔΔCT method. Reactions were performed in triplicate.
Cells were grown on two-well coated chamber slides. They were then fixed in 10% formalin for 20 minutes and washed in PBS three times for 5 minutes. Cells were permeabilized with 0.2% Triton X-100 (Sigma-Aldrich) in PBS at room temperature for 5 minutes. Following three additional PBS washes, cells were incubated in blocking buffer (PBS with 0.1% bovine serum albumin, BSA; Sigma-Aldrich) and 5% normal goat serum (Dako, Produktionsvej, Denmark) at room temperature for 1 hour. They were then incubated overnight with primary antibodies diluted in blocking buffer at 4℃. The primary antibody used in this analysis was rabbit anti-MAP 2 (1 : 100; Millipore, Temecula, CA, USA). Following three washes with PBS, cells were incubated in the dark with Alexa Fluor 594 goat anti-rabbit IgG (1 : 200; Invitrogen, Carlsbad, CA, USA) for 1 hour. Other antibodies included Alexa Fluor 555 conjugated mouse anti-β-tubulin III (1 : 50; BD Biosciences, Franklin Lakes, NJ, USA) and Alexa Fluor 488 conjugated mouse anti-GFAP (1 : 100; Millipore). Cell nuclei were stained with 1 µg/mL 4' ,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) at room temperature for 10 minutes. After mounting, fluorescent images were captured using confocal microscopy (Olympus America Inc., Center Valley, PA, USA).
Dental stem cells obtained from extracted human third molars (Fig. 1) had spindle shaped morphology, similar to the typical shapes of mesenchymal stem cells. After induction of neurogenic medium for 24 hours, the morphology changed into neurite-like cells, including cell processes. Most of the cells were similar in shape to neuronal cells, with very thin and long cytoplasmic processes, resembling axons, dendrites, and perikaryon.(Fig. 2)
Even though all of the cells showed expression of the neural markers, the differentiation group in neurogenic medium showed higher expression than the undifferentiated group (control).(Fig. 3. A) Also, the mRNA expression of the mature neuronal markers, NF-M and NSE, the central and peripheral glial marker, S100, and acetylcholine specific neuron marker, ChAT increased during induction.(Supplementary Fig. 1) Using real-time PCR, we quantified the mRNA expression levels of MAP2 and β-tubulin III as neuron markers and GFAP as an astrocyte marker in human SCAP, DPSCs, and PDLSCs.(Fig. 3. B) Upon neurogenic induction, real-time PCR results showed that mRNA levels had a tendency to increase for MAP2, β-tubulin III, and GFAP. Specifically, the expression of β-tubulin III increased in SCAP and PDLSCs compared with control cells (1.6-fold [P<0.01] and 1.4-fold [P<0.05], respectively; Student's t-test). GFAP mRNA level was significantly increased in DPSCs (2.3-fold [P<0.0001]; Student's t-test) and PDLSCs (1.5-fold [P<0.005]; Student's t-test), whereas the level appeared to remain unchanged in SCAP. On the basis of mRNA levels, our data suggest that SCAP and PDLSCs can be differentiated into neural cells, whereas DPSCs may be more apt to differentiate into glial cells than neural cells.
The mature neuronal marker, MAP2, was expressed throughout the cytoplasm and microtubules of cells that had undergone neuronal differentiation. The proportion of MAP2-positive cells after neurogenic induction was 88.9%, 75%, and 100% in SCAP, DPSCs, and PDLSCs, respectively. The expression of intermediate neuronal marker, β-tubulin III, was 66.7%, 50%, and 58.3% in SCAP, DPSCs, and PDLSCs, respectively, under the same conditions, compared to the control. In contrast to MAP2 and β-tubulin III, the protein expression of GFAP was not detected in either the control or the differentiation group.(Fig. 4) These data suggest that the neurogenic differentiation potential of SCAP and PDLSCs may be superior to that of DPSCs.
The aim of the present study was to characterize neurogenic differentiation of human dental stem cells. So far, cell therapy for neurological diseases has been studied using mesenchymal stem cells derived from bone marrow or adipose tissue, embryonic stem cells, and neural stem cells17. The nervous system consists of two types of cells, neurons and glial cells. Currently, the functional involvement of these cells in neurotransmission is not clearly understood. We hypothesized that dental stem cells, which originate from neural crest cells, may also be valuable candidates for the treatment of neurological diseases and for the repair of neuronal damage. Specifically, these cells were expected to be able to be utilized to repair dental nerves damaged during tooth extraction or implant procedures in the dental field.
The current study showed changes in phenotype, RNA levels, and protein expression in human SCAP, DPSCs, and PDLSCs due to neurogenic differentiation treatment. We used commercial neurogenic differentiation medium produced by PromoCell for neurogenic induction, because although various neurogenic differentiation methods have been proposed, optimal conditions have not yet been established. There are several other methods that have been used for neurogenic differentiation, such as chemical treatment, neurosphere induction, and growth factor-induced differentiation18,19,20.
Even after neural induction for only 24 hours, morphologic changes had taken place in almost all dental mesenchymal cells; these changes included extended neurite-like processes, which resemble dendrites and axons. Intermediate neuronal marker (β-tubulin III), mature neuronal marker (MAP2), and glial marker (GFAP) were used to analyze the neurogenic potential of SCAP, DPSCs, and PDLSCs. RT-PCR results showed that MAP2 mRNA level tended to increase in the differentiated groups compared with undifferentiated groups, though the differences were not statistically significant. The RNA level of β-tubulin III significantly increased within SCAP and PDLSCs in neurogenic differentiation medium but not in DPSCs. DPSCs showed a significantly increased GFPA mRNA expression under differentiation conditions, whereas SCAP expressed very little GFAP in both control and differentiation groups. Interestingly, MAP2 and β-tubulin III mRNAs were also found in undifferentiated conditions. These results suggest that these cells might have a neural origin and thus have a capacity for neurogenic differentiation. Moreover, DPSCs are prone to differentiate into glial cells rather than neurons. In terms of protein level, MAP2 was strongly expressed in SCAP, DPSCs, and PDLSCs after neurogenic induction; more than 75% of cells were MAP2-positive. The protein expression of β-tubulin III increased in dental stem cells under differentiation conditions to about 50%-70% of cells, whereas GFAP was not detected. However, the expressions of neuronal markers in DPSCs were lower than those in SCAP and PDLSCs. These results indicate that SCAP and PDLSCs may have a greater capacity to differentiate into neuronal cells than DPSCs.
Recently, the transplantation of human DPSCs was reported to improve motor capacity in a mouse spinal cord injury model, and stem cells from human exfoliated deciduous teeth promoted locomotor recovery following transection of rat spinal cords21,22. Indeed, DPSCs have been reported to not only produce neurotrophic factors for supporting the survival of neuronal cells, but also support the homing of endogenous neural stem cells to the injury site during transplantation23,24. In previous reports, transplantation experiments for nerve regeneration utilized DPSCs. In this report, we conclude that PDLSCs and SCAP may be more useful as cell sources for neuronal regeneration, because PDLSCs and SCAP express neural cell markers at higher levels than do DPSCs under neuronal differentiation culture conditions. Therefore, PDLSCs and SCAP, as well as DPSCs, may be appropriate cell-sources for neuronal regeneration, though further functional studies, such as in vivo tests or preclinical trials, are needed to reach a definite conclusion.
Notes
References
1. Duan X, Tu Q, Zhang J, Ye J, Sommer C, Mostoslavsky G, et al. Application of induced pluripotent stem (iPS) cells in periodontal tissue regeneration. J Cell Physiol. 2011; 226:150–157. PMID: 20658533.

2. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007; 100:1249–1260. PMID: 17495232.

3. Jones E, Yang X. Mesenchymal stem cells and bone regeneration: current status. Injury. 2011; 42:562–568. PMID: 21489533.

4. Malliaras K, Marbán E. Cardiac cell therapy: where we've been, where we are, and where we should be headed. Br Med Bull. 2011; 98:161–185. PMID: 21652595.

5. Ohba S, Ikeda T, Kugimiya F, Yano F, Lichtler AC, Nakamura K, et al. Identification of a potent combination of osteogenic genes for bone regeneration using embryonic stem (ES) cell-based sensor. FASEB J. 2007; 21:1777–1787. PMID: 17317722.

6. Dangaria SJ, Ito Y, Walker C, Druzinsky R, Luan X, Diekwisch TG. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation. 2009; 78:79–90. PMID: 19433344.

7. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA. 2000; 97:13625–13630. PMID: 11087820.
8. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci USA. 2003; 100:5807–5812. PMID: 12716973.

9. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004; 364:149–155. PMID: 15246727.

10. Lee JH, Um S, Jang JH, Seo BM. Effects of VEGF and FGF-2 on proliferation and differentiation of human periodontal ligament stem cells. Cell Tissue Res. 2012; 348:475–484. PMID: 22437875.

11. Um S, Choi JR, Lee JH, Zhang Q, Seo B. Effect of leptin on differentiation of human dental stem cells. Oral Dis. 2011; 17:662–669. PMID: 21702867.

12. Sonoyama W, Liu Y, Fang D, Yamaza T, Seo BM, Zhang C, et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS One. 2006; 1:e79. PMID: 17183711.

13. Komada Y, Yamane T, Kadota D, Isono K, Takakura N, Hayashi S, et al. Origins and properties of dental, thymic, and bone marrow mesenchymal cells and their stem cells. PLoS One. 2012; 7:e46436. PMID: 23185234.

14. Chai Y, Jiang X, Ito Y, Bringas P Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000; 127:1671–1679. PMID: 10725243.

15. Abe S, Hamada K, Miura M, Yamaguchi S. Neural crest stem cell property of apical pulp cells derived from human developing tooth. Cell Biol Int. 2012; 36:927–936. PMID: 22731688.

16. Karaöz E, Demircan PC, Sağlam O, Aksoy A, Kaymaz F, Duruksu G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol. 2011; 136:455–473. PMID: 21879347.

17. Kim SU, de Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009; 87:2183–2200. PMID: 19301431.

18. Völlner F, Ernst W, Driemel O, Morsczeck C. A two-step strategy for neuronal differentiation in vitro of human dental follicle cells. Differentiation. 2009; 77:433–441. PMID: 19394129.

19. Nourbakhsh N, Soleimani M, Taghipour Z, Karbalaie K, Mousavi SB, Talebi A, et al. Induced in vitro differentiation of neural-like cells from human exfoliated deciduous teeth-derived stem cells. Int J Dev Biol. 2011; 55:189–195. PMID: 21671222.

20. Aanismaa R, Hautala J, Vuorinen A, Miettinen S, Narkilahti S. Human dental pulp stem cells differentiate into neural precursors but not into mature functional neurons. Stem Cell Discov. 2012; 2:85–91.

21. Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest. 2012; 122:80–90. PMID: 22133879.

22. de Almeida FM, Marques SA, Ramalho Bdos S, Rodrigues RF, Cadilhe DV, Furtado D, et al. Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J Neurotrauma. 2011; 28:1939–1949. PMID: 21609310.

23. Nosrat IV, Smith CA, Mullally P, Olson L, Nosrat CA. Dental pulp cells provide neurotrophic support for dopaminergic neurons and differentiate into neurons in vitro; implications for tissue engineering and repair in the nervous system. Eur J Neurosci. 2004; 19:2388–2398. PMID: 15128393.

24. Arthur A, Shi S, Zannettino AC, Fujii N, Gronthos S, Koblar SA. Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells. 2009; 27:2229–2237. PMID: 19544412.

Supplementary Materials
Supplementary Fig. 1
The mRNA expression of neurofilament-medium chain (NF-M) and neuron-specific enolase (NSE) as mature neuronal markers differentially increased during induction, even though these markers were detected in non-induced cells. The gene expression of S100 as the central and peripheral glial marker and choline acetyltransferase (ChAT) as an acetylcholine-specific neuron marker had a tendency to increase in differentiation groups; the levels of these markers obviously increased in dental pulp stem cells (DPSCs). (GAPDH: glyceraldehyde 3-phosphate dehydrogenase, SCAP: stem cells from apical papilla, PDLSCs: periodontal ligament stem cells)
Fig. 1
Human third molars provide three different cell sources. A. Apical papilla from the developing root-tip was gently separated from the surface of the root (arrow). B. Dental pulp was isolated from the cracked crown (arrow). C. The periodontal ligament was removed from the middle root surface (arrow).

Fig. 2
Morphological changes of the humand dental stem cells were noticeable during induction of neurogenic differentiation. Upon stimulation by neurogenic differentiation medium, spindle-shaped cells changed into neuron-like cells, as identified via microscopy. Arrows indicate neurogenic differentiation of dental stem cells that extended neurite-like projections. Scale bars=100 µm. (SCAP: stem cells from apical papilla, DPSCs: dental pulp stem cells, PDLSCs: periodontal ligament stem cells)
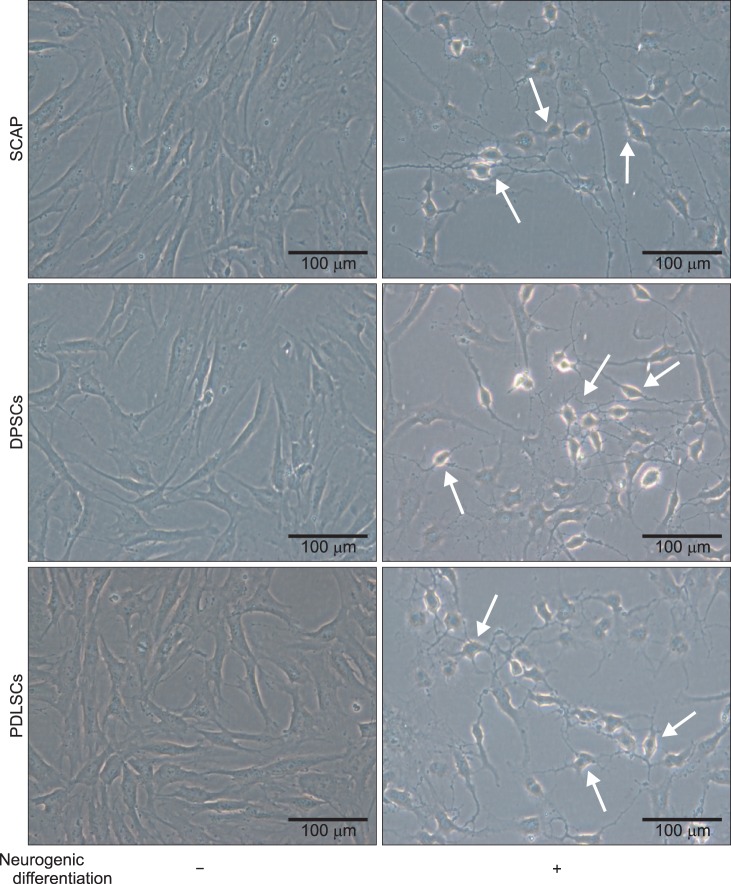
Fig. 3
Differentiated gene expression of dental stem cells upon neurogenic induction analyzed by reverse transcriptase polymerase chain reaction (RT-PCR) (A), and real-time PCR (B) of microtubule-associated protein 2 (MAP2), β-tubulin III, and glial fibrillary acidic protein (GFAP) genes. Neuron markers (MAP2 and β-tubulin III) were upregulated with various intensities under basal conditions. The expression of GFAP strongly increased in dental pulp stem cells (DPSCs) after stimulation by induction medium. Neuronal markers increased as neurogenic differentiation progressed, though MAP2 did not significantly increase compared to the control. Expression of β-tubulin III increased in stem cells from apical papilla (SCAP) and periodontal ligament stem cells (PDLSCs), while the mRNA level of GFAP increased significantly in DPSCs and PDLSCs after induction of neurogenic differentiation. Results are expressed as fold-change value relative to the normalized glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene. *P<0.05, **P<0.01, ***P<0.001.

Fig. 4
Immunofluorescence staining for neural markers after neurogenic induction. Microtubule-associated protein 2 (MAP2) and β-tubulin III were expressed in stem cells from apical papilla (SCAP), dental pulp stem cells (DPSCs), and periodontal ligament stem cells (PDLSCs) upon culturing in neurogenic medium; however, cells in neurogenic differentiation culture conditions did not express glial fibrillary acidic protein (GFAP). Scale bars=20 µm.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download