Abstract
Objectives
The purpose of this study was to evaluate the sinus bone graft resorption over 3 years after two-stage implant placement.
Materials and Methods
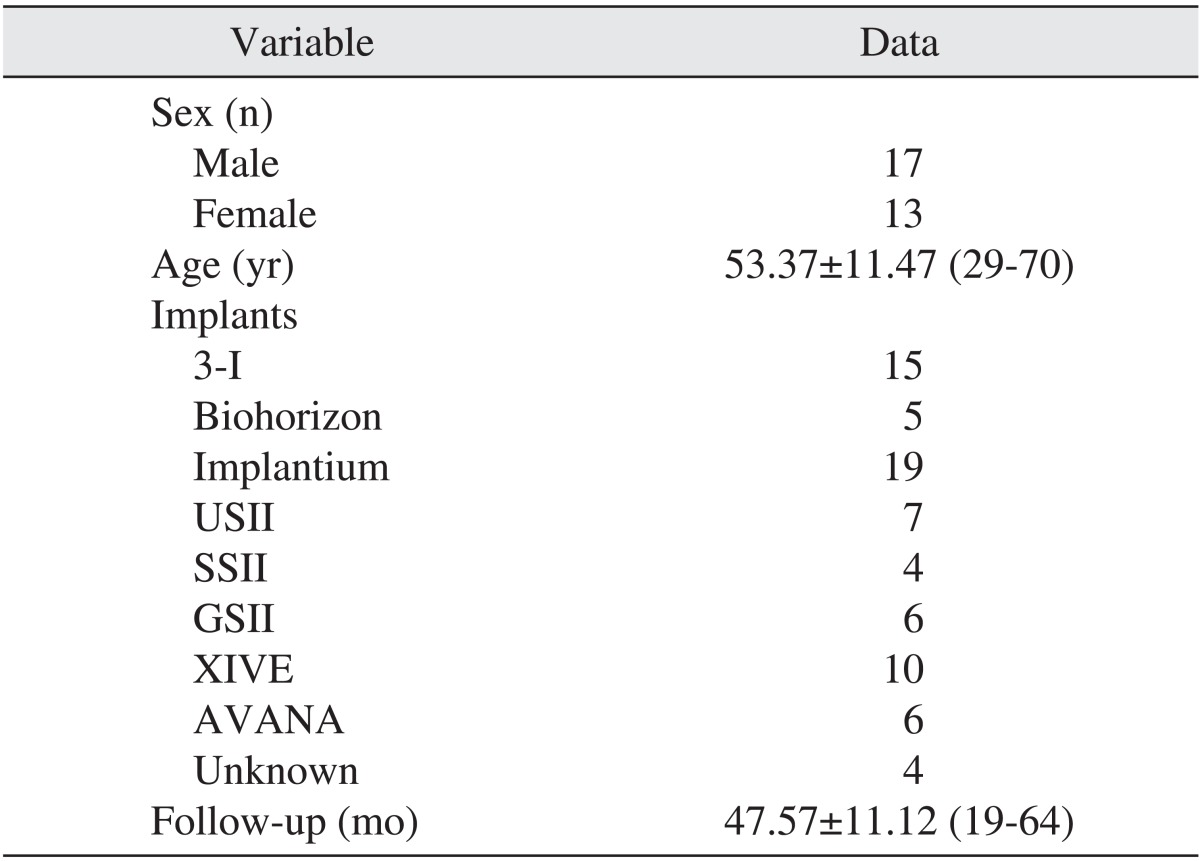
The subjects for this study included 30 patients whose maxillary posterior ridges were too atrophic for implants. Bone-added osteotome sinus floor elevation was used in 15 maxillary sinuses, while the bone graft by lateral approach technique was used in 25 maxillary sinuses. The height from the top of the fixture to the sinus floor was estimated immediately after implant placement and the follow-up period was over 3 years. The surgery was classified with two groups: sinus bone grafting with and without autogenous bone. All implants were placed simultaneously.
The edentulous posterior maxilla is considered to be a difficult area for placement of implants due to the atrophic alveolar bone, poor bone quality, and sinus pneumatization. Recently, with the development implant surface treatment, diverse bone graft materials and procedures, such anatomical hindrances have been overcome, and sinus bone grafting has been accepted as a common predictable treatment procedure.
Hatano et al.1 performed the Schneiderian membrane elevation by forming a lateral window, using bone graft materials mixed with Bio-Oss and autogenous bones at a ratio of 1 : 2, and placed implants. In their study, maxillary pneumatization occurred continuously for 2-3 years; thus, the grafted bone was resorbed. After 5-6 years, bone resorption progressed to the previous height of the sinus floor prior to sinus augmentation.
On the other hand, Zijderveld et al.2 reported a similar procedure using autogenous bones and tricalcium phosphate, performed delayed implant placement and evaluated the amount of long-term bone resorption. In their study, during the initial 1.5 years both groups showed significant vertical bone resorption; afterward, they showed very minute changes and stabilization of the bone. Therefore, prognosis after maxillary sinus bone graft procedure as assessed by long-term follow-up observation is still controversial, and a clear conclusion has not yet been made.
Furthermore, previous studies were conducted on cases where the lateral approach was performed. For cases with greater than 5-mm residual maxillary sinus bones, sinus membrane elevations using a crestal approach such as the bone-added osteotome sinus floor elevation (BAOSFE) have commonly been performed; nonetheless, studies comparing these procedures are very rare3.
The aim of this study is to evaluate and compare the amount of resorption of sinus bone graft materials following approach technique and use of autogenous bones as well as immediate the effect of presurgical and postsurgical complications on bone resorption.
From December 2003 to October 2005, sinus augmentation and immediate implant placement were performed simultaneously by one oral and maxillofacial surgeon at Seoul National University Bundang Hospital. More than 3 years after surgery, the cases were retrospectively analyzed. A total of 30 patients were recruited as study subjects, 76 implants were placed after maxillary sinus bone grafting, and the average follow-up time was 47.57±11.2 months.(Table 1) The sinus bone graft was classified as either a lateral or crestal approach. None of the patients had systemic diseases such as uncontrolled hypertension, diabetic mellitus, liver and kidney disease, or an autoimmune disease that may have exerted an effect on surgery; smoking and parafunctional habit status of the patients were not evaluated in our study. We got approval of Seoul National University Bundang Hospital Institutional Review Board (CDMDIRB-1320-104).
One oral and maxillofacial surgeon selected the surgical procedure considering both height and quality of the residual bone. Maxillary sinus bone grafts using lateral window technique and delayed implant placement was done in cases with less than 6 mm of residual bone height. BAOSFE was done in cases with more than 6 mm of residual bone height. In 25 maxillary sinuses, 51 implants were placed using the lateral approach, and in 15 maxillary sinuses, 25 implants were placed using the crestal approach. Local anesthesia, general anesthesia, or sedation were chosen according to the range of the surgery as well as the age and general condition of patients.
The mucoperiosteal flap was elevated by performing a crestal and bilateral vertical releasing incision. A rectangular or oval window was formed on the sinus anterior wall using a surgical round bur. The bony window was removed, and the sinus membrane was elevated. For cases that the sinus membrane was perforated during elevation, it was closed with the Surgicel (Johnson & Johnson, Gargrave, UK), Collatape (Zimmer Dental, Carlsbad, CA, USA), or resorbable collagen membranes according to its size. The sinus cavity was filled with bone graft materials, implants were placed, and the lateral window was covered with resorbable membranes.
The crestal incision was performed, and the mucoperiosteal flap was minimally elevated. Sinus lifting was performed according to the method suggested by Summers4. Drilling was performed, the sinus floor was elevated with an osteotome, and implants were placed after bone graft.
Bone materials were mixed and used, and they were divided into two groups, containing autogenous bones and without autogenous bones. In the group containing autogenous bones (46 implants), the bone harvested from the maxillary tuberosity or the mandible symphysis was mixed with allogeneic bones such as Regenaform (Exactech, Gainesville, FL, USA) or xenogeneic bones such as Bio-Oss (Geistlich Pharma AG, Wolhusen, Switzerland) and grafted. In the group without autogenous bones (30 implants), allogeneic bones such as Regenaform were mixed with xenogeneic bones such as Bio-Oss and grafted.
Panoramic radiographs were taken and the vertical height of maxillary bones was measured three times: (1) immediately after surgery, (2) after the initiation of prosthetic loading, and (3) during the final follow-up observation.
The distance between the supreme height of grafted maxillary sinus and the middle of the implant fixture was calculated. The amount of grafted bone resorption was defined as the difference in the bone height above the placed implant measured immediately after the bone graft and measured at the final follow-up observation.(Fig. 1) The gap of magnification ratio was reduced by calculating the real height of the placed implant and the measured height of the implant in the panoramic image.(Fig. 2)
Upon examination of medical records, complications such as intraoperative sinus perforation, postoperative wound dehiscence, infection, and osseointegration failure were observed.
The mean and standard deviation of the maxillary bone vertical height prior to surgery, immediately after surgery, and at final follow-up observation times were evaluated. Change in the bones grafted within the maxillary sinus caused by complications was also evaluated.
Differences in the resorption height of the grafted bone within the maxillary sinus according to bone graft procedure, and according to bone graft material, were both analyzed using independent sample t-tests; significance was evaluated at P<0.05. For statistical analyses, the PASW Statistics 17.0 (IBM Co., Armonk, NY, USA) software was used.
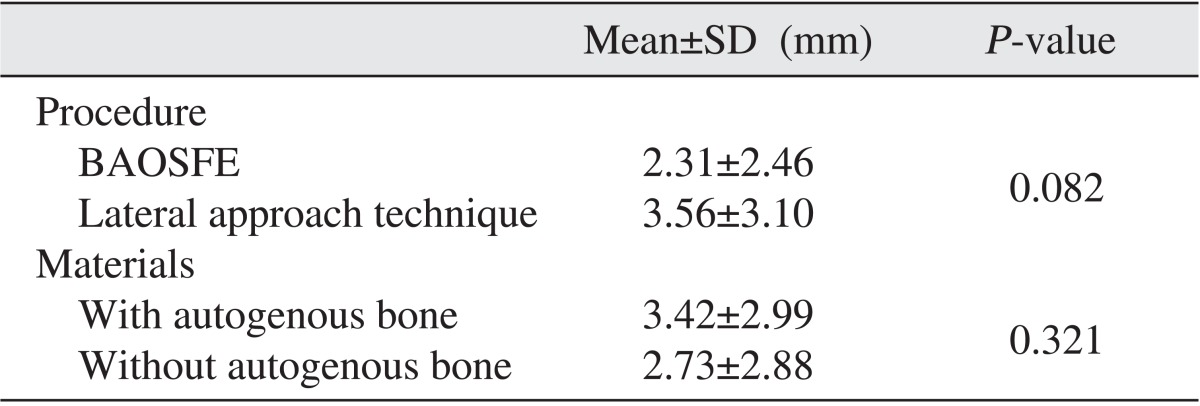
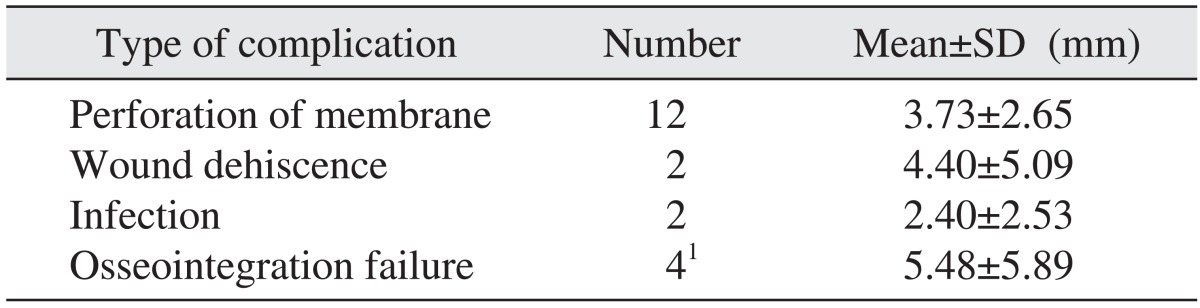
All implants were evaluated over 3 years and functional loading time was more than 24 months. The vertical height of the maxillary bone measured prior to surgery, immediately after surgery, and at the final follow-up observation was 6.47±2.82 mm, 18.37±4.82 mm and 15.22±5.10 mm, respectively. The resorption height of the maxillary bone at the final follow-up observation was 3.15±2.95 mm, and a significant difference was observed. When comparing surgical procedures, the resorption height using the crestal approach and lateral approach were 2.31±2.46 mm and 3.56±3.10 mm, respectively, but a significant difference between the two procedures was not detected (P=0.082). In regards to the bone graft materials, materials containing autogenous bones and materials without autogenous bones had resorption height of 3.42±2.99 mm and 2.73±2.88 mm, respectively, and a significant difference was not observed (P=0.321).(Table 2) Perforation of the maxillary sinus mucosa occurred in 12 cases, and the resorption height of the grafted bone was 3.73±2.65 mm, which was higher than the mean resorption height of the entire bones; nonetheless, a statistically significant difference was not shown. In addition, wound dehiscence and infection each developed in 2 cases, and the resorption heights of grafted bone were 4.40±5.09 mm and 2.40±2.53 mm respectively.(Table 3)
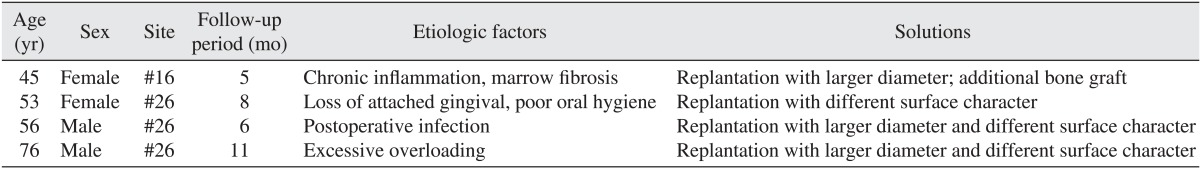
Among implants placed simultaneously with maxillary sinus bone grafts, removal of the implant due to the failure of osseointegration occurred in 4 implants, and the survival rate of implants was 94.7%. The mean age was 57.5±13.17 years, and these cases were associated with chronic inflammation in the maxillary sinus, postsurgical infection, destruction of the upper prosthesis speculated due to overloading, etc. The mean follow-up period to the failure of osseointegration or defect in the upper prosthesis was 7.50±2.65 months. All failed implants were removed and replaced. In the patients with failed implants, the resorption height of graft bone at the final follow-up time was 5.48±5.89 mm.(Tables 3, 4)
It is well known than even in atrophic maxillary bones, the pneumatized maxillary sinus is a contained defect that shows good results after bone grafting5,6. However, according to the study by Hatano et al.1, within the initial 2-3 years, the resorption of grafted bone progresses due to the re-pneumatization of the maxillary sinus, and although the resorption rate of grafted bone decreases gradually, it regresses to the vertical position of the initial maxillary bone. However, Block et al.7 reported that 73 implants were placed simultaneously with maxillary sinus bone graft, and 3 of them failed in the initial osseointegration. Nonetheless, at the final follow-up observation which was performed after 5-10 years, in approximately 90% of the 70 implants the grafted bone was well maintained in the area above the implants.
The representative xenogeneic bone, Bio-Oss, which is decalcified bovine bone, has been used as a maxillary sinus bone graft material for a long time8,9. It is believed that it delays the initial resorption of the bone that is grafted within the maxillary sinus, it is well maintained for a long time and is healed by the mechanism of osteoconduction10,11. Nonetheless, it has been pointed out that such bone graft materials which delay the resorption are inadequate for bone remodeling and functional loading of implants12,13. On the other hand, in maxillary sinus bone grafts, autogenous bones are inadequate for the long-term formation of bones due to rapid revascularization and resorption, injury of the donor area, and limitations in the harvest volume; however, some studies have shown that due to the osteogenesis capacity of cells within autogenous bones, with time they could provide osseointegration capacity to the implant surfaces1,14,15. In our study, an average resorption of 3.42 mm was shown when a mixture of autogenous bones and other bone graft materials were used, and an average resorption of 2.73 mm was shown when allogeneic bones and xenogeneic bones were used without the addition of autogenous bones.
Chanavaz16 reported that pneumatization of the maxillary sinus is induced by the formation of positive pressure within the maxillary sinus due to respiration. Hürzeler et al.17 reported that such positive pressure accelerates the resorption of grafted bones after maxillary sinus bone grafting. Different from the physiological atrophy process, the functional loading placed on implants may accelerate osteogenesis in the vicinity of implant, and in the initial period this may contribute to the stable maintenance of grafted bones after the resorption of grafted bone within the maxillary sinus18,19,20.
Wound dehiscence, acute and chronic sinusitis, and improper loading have been mentioned as contributing factors for failure of sinus bone grafts and implants. Bravetti et al.21 reported that during the physiological adaptation process after maxillary sinus bone grafting, chronic maxillary sinusitis can develop in the maxillary sinus mucosa. Wound dehiscence after implant placement may induce infection, delay wound healing, and induce the resorption of graft materials. In submerged implants, Mills22 observed a trend of increased resorption of the alveolar bone caused by the early development of wound dehiscence. Acute maxillary sinusitis that develops early after sinus augmentation induces erythematous swelling, pain, prulence, and fistula. Ultimately, it may act as the causative factor for the loss of grafted bone and bone resorption. The risk of infection is increased in cases with existing maxillary sinusitis or after maxillary sinus augmentation using a bone graft. Additionally, cytokines released from bacteria such as tumor necrosis factor-α and interleukins may induce rapid bone destruction through bone resorption23. Bone tissues around implant undergo continuous functional adaptive processes. Repeated loading may increase the level of tissue fatigue and induce microfissure of bone tissues; nonetheless, bone tissues spontaneously recover through bone remodeling processes, and homeostasis is maintained24.
In our study, osseointegration failed in 4 cases, and the destruction of the upper prosthesis was observed, suggesting chronic maxillary sinusitis as well as overloading. In one case, after the removal of an implant which had failed to osseointegrate, biopsy results showed bone marrow fibrosis, and we can speculate that the maxillary sinus bone graft materials did not mature. The large deviation of the resorption volume of grafted bone after wound dehiscence and the failure of osseointegration is thought to be due to the insufficient number of samples. Intrasurgical perforation of the maxillary sinus mucosa was associated with bone loss. In the cases with failure of osseointegration, it is speculated that the height of the residual alveolar bone prior to surgery was less than 2 mm; thus; excess bone grafting of more than 20 mm was performed, which induced a large deviation.
When such specific matters were excluded in the long-term observation, a great difference in the bone resorption rate between those cases with complications and those without was not observed. However, the cause of the failure to osseointegrate might be diverse, including chronic maxillary sinusitis, postsurgical infection, insufficient keratinized gingiva, poor oral hygiene, overloading, etc., and to resolve them, failing implants were switched to implants with a larger diameter that were treated with a sandblasting large-grit acid-etching technique and re-placed. If required, additional bone grafting was performed.
The limitations of our study include that it was a retrospective study that was performed using diverse bone graft materials and implant systems. Additionally, and in comparison with previous studies, the follow-up observation was short, and the number of cases was not sufficient.
In the future, prospective studies are needed to further elucidate these mechanisms. In particular, selective studies on factors that may mediate direct effects on wound healing as well as factors contributing to bone resorption, such as smoking, are required. In addition, the grafted bone within the maxillary sinus was measured in 2 dimensions; thus, the resorption amount and prognosis was evaluated under conditions that could not accurately reflect the amount of integrated bones in the vicinity of the implant, which could be a technical limitation. Therefore, supplemental studies are required in the future to further understand this system.
An evaluation of cases at an average of 47.6 months after maxillary sinus bone graft and implant placement showed that the average resorption volume of maxillary bone graft materials was 3.15±2.95 mm. Significant differences according to the bone graft procedure or materials were not observed. Complications that occurred during maxillary sinus bone graft such as perforation did not mediate the decisive effects on the resorption of grafted bones or the prognosis.
References
1. Hatano N, Shimizu Y, Ooya K. A clinical long-term radiographic evaluation of graft height changes after maxillary sinus floor augmentation with a 2:1 autogenous bone/xenograft mixture and simultaneous placement of dental implants. Clin Oral Implants Res. 2004; 15:339–345. PMID: 15142097.
2. Zijderveld SA, Schulten EA, Aartman IH, ten Bruggenkate CM. Long-term changes in graft height after maxillary sinus floor elevation with different grafting materials: radiographic evaluation with a minimum follow-up of 4.5 years. Clin Oral Implants Res. 2009; 20:691–700. PMID: 19453567.

3. Rosen PS, Summers R, Mellado JR, Salkin LM, Shanaman RH, Marks MH, et al. The bone-added osteotome sinus floor elevation technique: multicenter retrospective report of consecutively treated patients. Int J Oral Maxillofac Implants. 1999; 14:853–858. PMID: 10612923.
4. Summers RB. Sinus floor elevation with osteotomes. J Esthet Dent. 1998; 10:164–171. PMID: 9759033.

5. Kim YK, Yun PY, Kim SG, Kim BS, Ong JL. Evaluation of sinus bone resorption and marginal bone loss after sinus bone grafting and implant placement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e21–e28. PMID: 19138634.

6. Kim YK, Yun PY, Kim SG, Lim SC. Analysis of the healing process in sinus bone grafting using various grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:204–211. PMID: 18801669.

7. Block MS, Kent JN, Kallukaran FU, Thunthy K, Weinberg R. Bone maintenance 5 to 10 years after sinus grafting. J Oral Maxillofac Surg. 1998; 56:706–714. PMID: 9632328.

8. Yildirim M, Spiekermann H, Biesterfeld S, Edelhoff D. Maxillary sinus augmentation using xenogenic bone substitute material Bio-Oss in combination with venous blood. A histologic and histomorphometric study in humans. Clin Oral Implants Res. 2000; 11:217–229. PMID: 11168213.
9. Mayfield LJ, Skoglund A, Hising P, Lang NP, Attström R. Evaluation following functional loading of titanium fixtures placed in ridges augmented by deproteinized bone mineral. A human case study. Clin Oral Implants Res. 2001; 12:508–514. PMID: 11564112.
10. Piattelli M, Favero GA, Scarano A, Orsini G, Piattelli A. Bone reactions to anorganic bovine bone (Bio-Oss) used in sinus augmentation procedures: a histologic long-term report of 20 cases in humans. Int J Oral Maxillofac Implants. 1999; 14:835–840. PMID: 10612920.
11. Hallman M, Cederlund A, Lindskog S, Lundgren S, Sennerby L. A clinical histologic study of bovine hydroxyapatite in combination with autogenous bone and fibrin glue for maxillary sinus floor augmentation. Results after 6 to 8 months of healing. Clin Oral Implants Res. 2001; 12:135–143. PMID: 11251663.
12. Gosain AK. Hydroxyapatite cement paste cranioplasty for the treatment of temporal hollowing after cranial vault remodeling in a growing child. J Craniofac Surg. 1997; 8:506–511. PMID: 9477838.

13. Asai S, Shimizu Y, Ooya K. Maxillary sinus augmentation model in rabbits: effect of occluded nasal ostium on new bone formation. Clin Oral Implants Res. 2002; 13:405–409. PMID: 12175378.

14. Artzi Z, Weinreb M, Givol N, Rohrer MD, Nemcovsky CE, Prasad HS, et al. Biomaterial resorption rate and healing site morphology of inorganic bovine bone and beta-tricalcium phosphate in the canine: a 24-month longitudinal histologic study and morphometric analysis. Int J Oral Maxillofac Implants. 2004; 19:357–368. PMID: 15214219.
15. Aaboe M, Pinholt EM, Hjørting-Hansen E. Healing of experimentally created defects: a review. Br J Oral Maxillofac Surg. 1995; 33:312–318. PMID: 8555150.

16. Chanavaz M. Maxillary sinus: anatomy, physiology, surgery, and bone grafting related to implantology--eleven years of surgical experience (1979-1990). J Oral Implantol. 1990; 16:199–209. PMID: 2098563.
17. Hürzeler MB, Kirsch A, Ackermann KL, Quiñones CR. Reconstruction of the severely resorbed maxilla with dental implants in the augmented maxillary sinus: a 5-year clinical investigation. Int J Oral Maxillofac Implants. 1996; 11:466–475. PMID: 8803342.
18. Nyström E, Kahnberg KE, Albrektsson T. Treatment of the severely resorbed maxillae with bone graft and titanium implants: histologic review of autopsy specimens. Int J Oral Maxillofac Implants. 1993; 8:167–172. PMID: 8359872.
19. Keller EE, Eckert SE, Tolman DE. Maxillary antral and nasal one-stage inlay composite bone graft: preliminary report on 30 recipient sites. J Oral Maxillofac Surg. 1994; 52:438–447. PMID: 8169704.

20. Listrom RD, Symington JM. Osseointegrated dental implants in conjunction with bone grafts. Int J Oral Maxillofac Surg. 1988; 17:116–118. PMID: 3133419.

21. Bravetti P, Membre H, Marchal L, Jankowski R. Histologic changes in the sinus membrane after maxillary sinus augmentation in goats. J Oral Maxillofac Surg. 1998; 56:1170–1176. PMID: 9766543.

22. Mills MP. Spontaneous early exposure of submerged endosseous implants resulting in crestal bone loss: a clinical evaluation between stage I and stage II surgery. Implant Dent. 2003; 12:9–10.
23. Wang CY, Stashenko P. The role of interleukin-1 alpha in the pathogenesis of periapical bone destruction in a rat model system. Oral Microbiol Immunol. 1993; 8:50–56. PMID: 8510985.
24. Yeh CK, Rodan GA. Tensile forces enhance prostaglandin E synthesis in osteoblastic cells grown on collagen ribbons. Calcif Tissue Int. 1984; 36(Suppl 1):S67–S71. PMID: 6430525.

Fig. 1
Sinus bone resorption at long-term follow-up. The amount of resorption is defined as subraction of the final graft height (FGH) from the initial graft height (IGH). A. Right after sinus bone graft. B. At final follow-up.
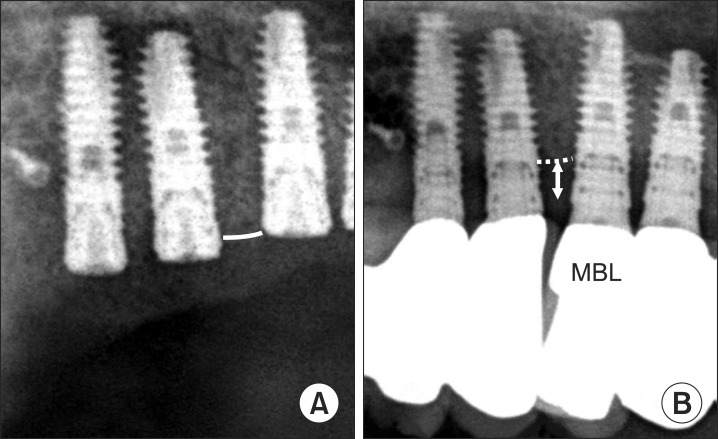
Fig. 2
Marginal bone loss (MBL) measurement. A. Right after sinus bone graft. B. At final follow-up.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download