Abstract
Objectives
The purpose of this study was to investigate the wound healing effect of primary cultured oral mucosal keratinocytes (OMKs) and to assess their roles in skin wounds.
Materials and Methods
OMK labeled with BromodeoxyUridine were scattered onto 1.5×1.5 cm skin defects of adult female nude mice (OMK group, n=15). For the control, culture media were placed on the wound (control group, n=15). Mice in both groups were sacrificed at three days (n=5), one week (n=5), and two weeks (n=5), and histomorphometric and immunoblot analyses with keratinocyte growth factor (KGF), interleukin (IL)-6, and IL-1α antibody were performed for the biopsied wound specimen. To verify the effect of the cytokine, rhIL-1α was applied instead of OMK transplantation, and the OMK and control groups were compared with regard to re-epithelialization.
Results
Histomorphometric analyses demonstrated faster re-epithelialization in the graft group than in the control group at the third day, first week, and second week. Newly forming epithelium showed maintenance of the histological character of the skin epithelium. The graft group showed superior expression of KGF, IL-6, and IL-1α protein, compared with the control group. Similar faster re-epithelialization was observed after treatment with rhIL-1α instead of OMK transplantation.
Complete regeneration of own tissue is the ideal treatment for tissue loss in the oral and maxillofacial region. Note, however, that self-regeneration is often hard to achieve. Therefore, various surgical reconstructions have been developed for functional restoration, which sometimes results in the morbidity of the unnecessary donor site and long-term complications. Moreover, mechanical devices cannot always restore the function of an organ completely1.
To overcome such disadvantages, new reconstructive modalities using tissue engineering have been investigated as alternative for these therapies. Tissue engineering is an interdisciplinary field that applies the principles of engineering and life science to the development of biologically functional constituents that restore, maintain, or improve tissue function2. Many investigations and clinical trials have been attempted to engineer virtually every type of mammalian tissue.
Since autologous keratinocyte sheets were used to treat burns in 19813, several improved methods have been established to make bioengineered skin substitutes using only extracellular matrix, mainly cells, or combination of cells and matrices4,5.
Trials to utilize the oral mucosa have been performed more recently6-9. Several types of composite grafts of cultured oral mucosal keratinocytes (OMKs) and fibroblasts of the lamina propria of the oral mucosa or dermal analog have been developed10-12. Note, however, that the preparation procedure of a composite tissue is complicated and time-consuming.
There are a few reports on the successful grafting of epi-thelial sheets from the oral mucosa for large intraoral wounds and skin repair9,13. Nonetheless, using only the epidermal component without the dermal matrix has several disadvantages such as fragility, wound contracture, and varying graft "take" rate12,14.
To overcome these disadvantages, the cell suspension technique was developed. The cell suspension technique is less time-consuming than the preparation of the sheet grafts, and it is now possible to transfer the proliferating single keratinocytes actively with a simplified application method7.
Cultured keratinocytes exhibit a hyperproliferative phenotype and release cytokines that are not found in stable condition15,16. The cytokines produced by the activated keratinocyte play a role in immunity, inflammatory reaction, control of cell growth and differentiation, and wound healing17,18.
The main objective of this investigation is to assess the role of suspension of cultured OMKs in skin wounds of athymic nude mice for the previous study19.
Human oral mucosal tissue was obtained from non-inflamed gingival tissue of 5 donors in their 20s who underwent surgical extraction of the third molar. Primary cultures of OMKs were performed on a collagen-coated plate (IWAKI, Japan) as described previously20. To trace the grafted cells by BrdU labeling, 20 µmol/L of BrdU each was added to the culture medium 3 days and 1 hour before cell transplantation. The cultured OMKs were examined under phase contrast microscope, and the labeled cells were detected using anti-BrdU immunohistochemistry.
A total of 30 adult female nude mice (Balb/c Slc-nu, Japan SLC, Inc.) 8 weeks of age and weighing 20±2 g were used for the OMK transplantation, with 15 mice for the control (CTL) group and the remaining 15 for the OMK group. All mice were injected with 25 mg of ketamine HCl (Hankookunite, Korea), pentobarbital sodium (Choongwae, Korea), and flumoxef sodium (Ildong, Korea) intraperitoneally. We created a 1.5×1.5 cm full-thickness wound and sutured the normal skin ends and underlying muscles of the defect wound with 5/0 Mersilk (Johnson & Johnson Intl, UK) at the corners and centers of each side to prevent wound contracture. Primary cultured OMKs labeled with BrdU were suspended in culture medium at a density of 1×105 cells/40 µL onto skin defects in the OMK group, and the culture medium without cells was placed in the CTL group. For the wound dressing, three pieces of lens cleaning tissue (Whatman, UK) coated with vaseline gauze (Daeshin, Korea) were placed over the skin defect area in both groups; the wound areas were then secured with Elatex (Alcare Co., Japan). Vaseline gauze could prevent the skin wound from drying, and Whatman lens cleaning tissue absorbs the excess grease from the vaseline gauze. Five mice for each group were sacrificed at the 3rd day, 1st week, and 2nd week from the date of OMK transplantation by being divided into groups named CTL-3rd day, 1st week, 2nd week, OMK-3rd day, 1st week, and 2nd week groups, respectively.
As a trace marker for grafted cells, BrdU was injected into the culture media before cell transplantation. Double immunohistochemical staining using anti-cytokeratin (CK) AE1/3 and anti-BrdU was performed in the paraffin section of the OMK-3rd day groups. Anti-CK AE1/3 was used to detect epithelial cells, and anti-BrdU immunohistochemistry, to detect the labeled cells. Anti-BrdU (Sigma, USA) was treated first, and color development was done with 3,3'-diaminobenzidine (DAB; Vector, USA). The sections were reblocked and treated with monoclonal mouse anti-human CK, Clones AE1/AE3 (Anti-CK AE1/3; Dako). 3-amino-9 ethyl carbazole (AEC; Vector) was reacted for color development for CK AE1/3. The sections were lightly counterstained with Mayer's hematoxylin. For negative control samples, phosphate buffered saline (PBS) was substituted for the primary antibody.
At the 3rd day, 1st week, and 2nd week since the OMK transplantation date, wounds were totally excised, including the visible surgical defect area and normal tissue surroundings. Samples were divided into halves. One piece was fixed and embedded in paraffin for the histological examination and histomorphometric analysis of the center of the wound; the other piece was used for western blot analysis to investigate cytokine expression. Serial sections 4 µm thick were used for H&E staining for the histological examination. In the H&E slides, the length of the regenerating epithelia was measured using Image-Pro Plus (Media Cybernetics, MD, USA). Each value was recorded in the dBase and analyzed using the SAS program (SAS Institute Inc.).
Serial five data per section were statistically analyzed using one-way ANOVA followed by paired t-test to compare the length of the regenerating epithelial front. P-value of less than 0.05 was considered to be statistically significant.
Cytokine expression and growth factors (keratinocyte growth factor [KGF], interleukin [IL]-1α, and IL-6) were determined using western blot analysis. The proteins were extracted from the excised tissues of both CTL and OMK groups after the 3rd day, 1st week, and 2nd week by RIPA buffer (Roche Applied Science, Indianapolis, IN, USA). 15 µg of proteins was separated in 10% SDS polyacrylamide gel, and then transferred onto a nitrocellulose membrane. The membrane was blocked with 10% dry milk in PBS and incubated with monoclonal anti-KGF (Santa Cruz Biotechnology), IL-1α (Santa Cruz Biotechnology), and IL-6 antibody (Santa Cruz Biotechnology). β-actin was used as loading control.
To verify the effect of cytokine, rhIL-1α (R&D) was applied instead of OMK transplantation. Three mice were used for each of the CTL and rhIL-1α treatment groups. As previously described, 1.5×1.5 cm skin wounds were made in the dorsal surface of the mice and sutured. 10 ng/mL of rhIL-1α was scattered onto the skin defect area in the IL-1α treatment group, with only the culture medium placed in the CTL group. Wound dressing was done in the same method with OMK transplantation. They were sacrificed 5 days after the treatment and divided into CTL-5rd day and rhIL-1α-5rd day groups.
The phase contrast microscopic findings revealed that cultured OMKs predominantly consist of uniform, small basaloid cells and intersperse with larger differentiated cells when confluent.(Fig. 1. A) The human OMKs in culture were positive for anti-BrdU before grafting.(Fig. 1. B) The cultured cells can be successively pursued after the graft with anti-BrdU.
Double immunohistochemical analysis for the regenerating epithelium and grafted OMKs was performed with anti-CK AE1/3 and anti-BrdU in OMK-3rd day groups. CK AE1/3 appeared in the cytoplasm of the entire epithelium except the basal and suprabasal cells. The transitional epithelium of the wound margin was thickened and was positive for anti-CK AE1/3 but negative for anti-BrdU. There was active re-epithelialization, but grafted OMKs were absent in the marginal zone of the wound.
Scattered in the mid-portion of the wound were epithelial cell nests showing both cytoplasmic CK AE1/3 and nuclear BrdU expression.(Fig. 1. C) Cultured and transplanted OMKs labeled with BrdU were present in the skin wound of OMK-3rd day groups, predominantly in the central zone.
The histological analysis showed the effect of the grafted OMKs on re-epithelialization. Within the 3rd day, the regeneration of epithelium from the wound margin toward the central defect significantly increased in the OMK group compared to the CTL group.(Figs. 2. A, 2. C) A few acute inflammatory cells and scattered cell nests were found in the central defect area of the OMK-3rd day group. The regenerating basal cells showed palisading alignment with polarity. There was a prickled cell layer, but no rete ridge was observed.(Fig. 2. A) After the 2nd week, complete epithelialization was observed in the OMK group, but not in the CTL group.(Figs. 2. B, 2. D) In the regenerating epithelium, polarity of normal basal cells and differentiation of squamous cells were observed, similar to normal skin. Most of the epithelium was orthokeratinized and focally parakeratinized. Fibrosis was also noted.(Fig. 2. D)
The histologically observed length between the margin of the wound and regenerating epithelial front was measured and calculated using Image-Pro Plus. The average length of the regenerating epithelium in the CTL-3rd day, CTL-1st week, and CTL-2nd week groups was 0.73 mm (9.7%), 1.88 mm (25.1%), and 2.99 mm (39.9%), respectively. The average length of the regenerating epithelium in the OMK-3rd day, OMK-1st week, and OMK-2nd week groups was 3.76 mm (50.1%), 6.64 mm (88.5%), and 7.5 mm (100%), respectively. The regenerating epithelial length increased in a time-dependent fashion, with the rate of re-epithelialization significantly faster in the OMK groups than the CTL groups (P<0.05).
We found that wound beds undergo staged response to the wound healing procedure by producing pro-inflammatory cytokines and growth factors. The expressions of KGF, IL-6, and IL-1α at various times after the graft of cultured OMKs were investigated. KGF produced by fibroblasts affects the step of epithelial proliferation in wound healing and stimulates keratinocyte migration, proliferation, and differentiation. As the cytokines produced by leucocytes, IL-1α and IL-6 are the most important mediators of the acute-phase inflammatory reaction. IL-1α stimulates the production of IL-6, forming a cascade of cytokines. IL-1α can induce fibroblast proliferation, which is also chemotactic for fibroblast, stimulating the synthesis of collagen. In the OMK groups of our study, the western blot analysis demonstrated superior expression of KGF, IL-6, and IL-1α over the CTL groups. The expression patterns of KGF, IL-6, and IL-1α were all different. The KGF level was maintained highly through the 3rd day to the 1st week but decreased on the 2nd week. IL-6 gradually increased after the 3rd day and peaked at the 1st week. The expression of IL-1α was at its maximum on the 3rd day and gradually decreased in the OMK groups but showed continual increase in the CTL groups.(Fig. 3)
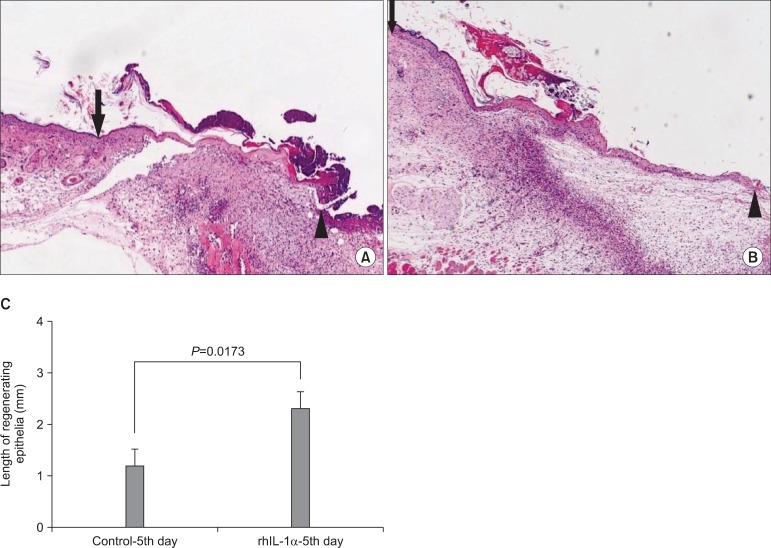
The average length of the regenerating epithelium of the CTL-5rd day and rhIL-1α-5rd day group was 1.17 mm and 2.29 mm, respectively. The marginal portion of the rhIL-1α treatment group showed faster re-epithelialization than the CTL group (P<0.0.5).(Fig. 4)
The reconstruction of the oral innate deficiency and maxillofacial surgical defects has been a primary target for regenerative medicine.
There are several advantages of using oral keratinocytes for wound healing instead of skin keratinocyte: First, oral keratinocyte has shorter turnover time with a resulting shorter culture time over skin keratinocyte. Second, cells can be maintained longer under culture conditions without terminal differentiation, whereas skin keratinocyte easily becomes keratinized in vitro, thereby losing its viability. Third, donor site morbidity is minimal because the biopsy scar of the oral cavity is inconspicuous9. Therefore, the autologous graft of cultured oral keratinocytes can avoid immunological problems and promote efficiency in the preparation and maintenance of cultured cells.
There are various systems to carry the cultured cells into a living recipient site. Recently, several trials for the three-dimensional reconstruction of the OMKs have been done21, revealing the following unsolved problems in clinical use: rolling of composites due to lack of physical property; difficulty of manipulation, and; graft contraction.
The cell suspension technique is less time-consuming in cell culture; carrying the cultured cells into the recipient site is also simpler than the preparation of sheet grafts or three-dimensional reconstruction. Thus, it has advantage over sheet grafts or composite grafts. Horch et al.22 developed a technique of single cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute and reported a successful result of reproducible, standardized full-thickness wounds in an athymic nude mouse model compared to cultured epidermal sheet grafts.
In this study, we examined the acceleration of re-epithelialization and inducement of skin epithelium by cultured and suspended OMKs in the skin wound regions of athymic nude mice. The histomorphometric evaluation revealed that the OMK groups showed more rapid re-epithelialization than the CTL groups. Such differences have statistically significant meaning, suggesting that primary cultured OMKs promote the re-epithelialization of skin wound defects.
Cultured and transplanted OMKs may provide factors that help the ingrowth of recipient skin keratinocytes from the wound edges unlike when they are implanted, since the differentiation of new forming epithelium shows mainly the orthokeratinization of cornified layer as the character of skin epithelium. Therefore, the grafted OMKs labeled with BrdU were present in the skin wound at immediate post-graft status but may undergo apoptosis later. Inflammation remained longer in the CTL groups than the OMK groups, and such may be related to the retardation of re-epithelialization.(Figs. 2. B, 2. D)
Mackenzie and Hill suggested the epitheliomesenchymal interaction (EMI), i.e., the differentiation and maintenance of epithelial specificity are directly dependent on the influence of the underlying mesenchymal tissue23,24. Based on EMI, the specificity of recipient epithelial cells is reproducible by grafting only epithelial cells without underlying connective tissue9. In this study, the maintenance of skin epithelial specificity of wound beds was enabled by grafting only OMKs without underlying connective tissue to the skin wound defect area. Therefore, the cell suspension technique has an additional advantage in functional reconstruction.
In normal mucosa and skin, there is balance between the number of keratinocytes being produced in the basal cell layer and the number of cells shed at the surface. The cultured keratinocytes exhibit a hyperproliferative phenotype. Gottlieb et al.15 and Kupper16 demonstrated that keratinocyte, when injured or cultured, releases a number of cytokines not found in stable epidermis and described it as "activated keratinocyte".
We hypothesized that grafted OMKs may induce cytokine production for accelerated wound healing. To prove this hypothesis, we analyzed the expression of KGF, IL-6, and IL-1α at different moments, such as 3rd day, 1st week, and 2nd week after the grafting of cultured OMKs. The OMK groups showed higher expression levels of KGF, IL-6, and IL-1α in comparison with the CTL groups. The expression patterns of KGF, IL-6, and IL-1α were quite different, suggesting that KGF, IL-6, and IL-1α appear at different times through the process of wound healing. Note, however, that cytokines can also be influenced by the extent of inflammation. The gradual increase of IL-1α level in the CTL groups may have been caused by continual inflammation due to retarded wound healing.
To verify the role of the cytokine for re-epithelialization, rhIL-1α was added externally to the skin wound of the nude mouse instead of the OMK graft. The marginal portion of the rhIL-1α treatment group showed faster re-epithelialization than the CTL group. This result suggests that the released cytokines produced by the graft of the OMKs play a main role in the acceleration of re-epithelialization of the wound. In conclusion, grafted OMKs accelerate the epithelial regeneration of skin wounds through the stimulation of cytokine expression.
Maas-Szabowski et al.25,26 demonstrated that keratinocyte growth regulation in organotypic co-cultures is mainly regulated by keratinocyte-derived IL-1 signaling, which induces KGF expression in co-cultured fibroblasts. The increased cytokine expression of the OMK groups in our experiment may depend on the reciprocal activation between recipient cells and grafted OMKs.
The cell suspension has a limitation in terms of the wound site for application because it is affected by gravity. Fibrin glue is one of the candidates for the appropriate carrier of cell suspension. It is technically easy to use, involving less tissue trauma in manipulation. The glue has high concentration of fibrinogen and growth factors, inducing rapid wound healing. To eliminate the effect of fibrin glue on wound healing, we did not use it as carrier. Horch et al.22 suspended the cultured cell pellet in fibrin glue with heterogenous human plasma protein fractions. Recently, good clinical records for cell graft with autologous fibrin glue were reported27. Autologous fibrin glue contains large numbers of platelets releasing significant quantities of growth factors that are known to promote wound healing28. Note, however, that this technique has a drawback, i.e., requires an additional process in making autologous fibrin glue from the patient's own blood.
The use of an appropriate carrier could be helpful in overcoming the limitation of cell suspension technique, inducing more rapid wound healing not only by the retention of more grafted cells in the wounds but also through the release of more cytokines and growth factors.
References
3. O'Connor NE, Mulliken JB, Banks-Schlegel S, Kehinde O, Green H. Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981; 1:75–78. PMID: 6109123.
4. Eaglstein WH, Falanga V. Tissue engineering for skin: an update. J Am Acad Dermatol. 1998; 39:1007–1010. PMID: 9843017.

5. Lee KH. Tissue-engineered human living skin substitutes: development and clinical application. Yonsei Med J. 2000; 41:774–779. PMID: 11204828.

6. Lauer G. Autografting of feeder-cell free cultured gingival epithelium. Method and clinical application. J Craniomaxillofac Surg. 1994; 22:18–22. PMID: 8175992.
7. Lauer G, Schimming R. Tissue-engineered mucosa graft for reconstruction of the intraoral lining after freeing of the tongue: a clinical and immunohistologic study. J Oral Maxillofac Surg. 2001; 59:169–175. PMID: 11213985.

8. Lauer G, Schimming R, Frankenschmidt A. Intraoral wound closure with tissue-engineered mucosa: new perspectives for urethra reconstruction with buccal mucosa grafts. Plast Reconstr Surg. 2001; 107:25–33. PMID: 11176597.

9. Ueda M, Hata K, Horie K, Torii S. The potential of oral mucosal cells for cultured epithelium: a preliminary report. Ann Plast Surg. 1995; 35:498–504. PMID: 8579268.

10. Imaizumi F, Asahina I, Moriyama T, Ishii M, Omura K. Cultured mucosal cell sheet with a double layer of keratinocytes and fibroblasts on a collagen membrane. Tissue Eng. 2004; 10:657–664. PMID: 15265283.

11. Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003; 32:188–197. PMID: 12729781.

12. Izumi K, Feinberg SE, Terashi H, Marcelo CL. Evaluation of transplanted tissue-engineered oral mucosa equivalents in severe combined immunodeficient mice. Tissue Eng. 2003; 9:163–174. PMID: 12625965.

13. Lauer G, Schimming R, Gellrich NC, Schmelzeisen R. Prelaminating the fascial radial forearm flap by using tissue-engineered mucosa: improvement of donor and recipient sites. Plast Reconstr Surg. 2001; 108:1564–1572. discussion 73-5. PMID: 11711928.

14. Cooper ML, Andree C, Hansbrough JF, Zapata-Sirvent RL, Spielvogel RL. Direct comparison of a cultured composite skin substitute containing human keratinocytes and fibroblasts to an epidermal sheet graft containing human keratinocytes on athymic mice. J Invest Dermatol. 1993; 101:811–819. PMID: 8245510.

15. Gottlieb AB, Chang CK, Posnett DN, Fanelli B, Tam JP. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988; 167:670–675. PMID: 3279155.

16. Kupper TS. The activated keratinocyte: a model for inducible cytokine production by non-bone marrow-derived cells in cutaneous inflammatory and immune responses. J Invest Dermatol. 1990; 94:146S–150S. PMID: 2141048.
17. Andreadis ST, Hamoen KE, Yarmush ML, Morgan JR. Keratinocyte growth factor induces hyperproliferation and delays differentiation in a skin equivalent model system. Faseb J. 2001; 15:898–906. PMID: 11292649.

18. McKay IA, Leigh IM. Epidermal cytokines and their roles in cutaneous wound healing. Br J Dermatol. 1991; 124:513–518. PMID: 2064935.

19. Kim HS, Kim NH, Kim J, Cha IH. The inductive capacity of primary cultured oral mucosal keratinocytes in skin wound healing of athymic nude mice. J Korean Assoc Oral Maxillofac Surg. 2004; 30:308–315.
20. Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: the formation of keratinizing colonies from single cells. Cell. 1975; 6:331–343. PMID: 1052771.
21. Cha IH, Yook JI, Son YS, Lee EH, Jeong SY, Kim KJ, et al. Three dimensional reconstitution of oral mucosal keratinocytes and its biologic characteristics. Korean J Pathol. 2000; 34:181–189.
22. Horch RE, Bannasch H, Kopp J, Andree C, Stark GB. Single-cell suspensions of cultured human keratinocytes in fibrin-glue reconstitute the epidermis. Cell Transplant. 1998; 7:309–317. PMID: 9647440.

23. Hall BK. A role for epithelial-mesenchymal interactions in tail growth/morphogenesis and chondrogenesis in embryonic mice. Cells Tissues Organs. 2000; 166:6–14. PMID: 10671750.

24. Mackenzie IC, Hill MW. Connective tissue influences on patterns of epithelial architecture and keratinization in skin and oral mucosa of the adult mouse. Cell Tissue Res. 1984; 235:551–559. PMID: 6201277.

25. Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999; 112:1843–1853. PMID: 10341204.

26. Maas-Szabowski N, Stark HJ, Fusenig NE. Keratinocyte growth regulation in defined organotypic cultures through IL-1-induced keratinocyte growth factor expression in resting fibroblasts. J Invest Dermatol. 2000; 114:1075–1084. PMID: 10844548.

27. Lendeckel S, Jodicke A, Christophis P, Heidinger K, Wolff J, Fraser JK, et al. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: case report. J Craniomaxillofac Surg. 2004; 32:370–373. PMID: 15555520.

28. Thorn JJ, Sorensen H, Weis-Fogh U, Andersen M. Autologous fibrin glue with growth factors in reconstructive maxillofacial surgery. Int J Oral Maxillofac Surg. 2004; 33:95–100. PMID: 14690664.

Fig. 1
Evaluation of the cultured cells. A. The phase contrast image showed that cultured oral mucosal keratinocytes (OMKs) predominantly consisted of uniform, small basaloid cells and interspersed with larger differentiated cells when confluent. B. After 25 hours of cultivation of OMKs with BromodeoxyUridine (BrdU), all of the cells revealed positive reaction to anti-BrdU immunohistochemical stain. The cultured cells can be successively pursued after the graft with anti-BrdU. (immunohistochemical staining for anti-BrdU, DAB; ×100) C. There were scattered epithelial cell nests in the mid-portion of the wound of OMK-3rd day groups. The cytoplasm of the cells was positive for anti-cytokeratin (CK) AE1/3, and the nucleus of the cells was positive for anti-BrdU. The arrow head indicates its nuclei, which proves that cultured and transplanted cells are present in the graft. (double immunohistochemical staining for anti-BrdU and anti-CK AE1/3, DAB, and AEC; ×200) Arrow head: nuclei of graft cell showing a positive result for anti-BrdU.
Cited from Fig. 3. B in the article of Kim HS, et al. [J Korean Oral Maxillofac Surg 2004;30:308-315].

Fig. 2
Histological analysis of re-epithelialization. (H&E stainig) A. The control-3rd day group showed minimal regeneration of the epithelial front from the wound margin toward the central defect. The central portion of the defect showed an abundance of inflammatory cell infiltrates. (×100) B. The center of the control-2nd week group was uncovered with epithelial cells. Diffuse infiltrates of inflammatory cells in the connective tissue were noted. (×40) C. The oral mucosal keratinocytes (OMK)-3rd day group showed active regeneration of epithelium from the wound margin toward the central defect. A few acute inflammatory cells were found in the central defect area. The regenerating basal cells showed palisading alignment with polarity. A prickled cell layer appeared, but the rete ridge was not found. (×40) D. The central portion of the OMK-2nd week group showed complete epithelialization. The regenerating epithelium showed the polarity of normal basal cells and the differentiation of squamous cells. Most of the epithelium was orthokeratinized and focally parakeratinized. Minor inflammation and fibrosis were also observed. (×40) Arrows: wound margin, arrow heads: regenerating epithelial front.
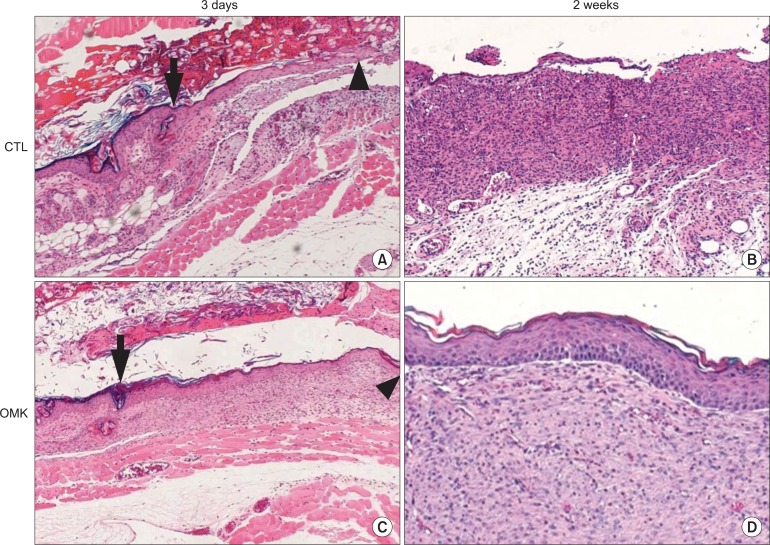
Fig. 3
Western blot analysis of keratinocyte growth factor (KGF), interleukin (IL)-6, and IL-1α expression at various times after the graft of cultured oral mucosal keratinocyte (OMK). The western blot analysis demonstrated superior expression of KGF, IL-6, and IL-1α in OMK groups over the control groups. In the OMK groups, the expression patterns of KGF, IL-6, and IL-1α were quite different. KGF level was maintained highly through the 3rd day to the 1st week, decreasing at the 2nd week. IL-6 gradually increased after the 3rd day and peaked at the 1st week. IL-1α peaked at the 3rd day and gradually decreased after the 3rd day. In the control groups, IL-1α level increased gradually KGF, IL-6, IL-1α.

Fig. 4
Evaluation of the effect of rhIL-1α treatment on re-epithelialization. To verify the effect of cytokine, 10 ng/mL rhIL-1α was applied to the skin wound of the nude mouse instead of the oral mucosal keratinocyte (OMK) graft. The marginal portion of the rhIL-1α treatment group showed faster re-epithelialization than the control group. A. Control-5th day groups, B. rhIL-1α treatment groups. (H&E staining, ×40) C. Measurement of the regenerating epithelia. The average length of the regenerating epithelial front of the control-5th day and rhIL-1α-5th day groups was 1.17 mm and 2.29 mm, respectively.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download