Abstract
Artecoll (Artes Medical Inc., San Diego, CA, USA) has recently been developed as a permanent synthetic cosmetic filler. We experienced an inflammatory granuloma resulting from a previous injection of Artecoll at the upper lip, which was regarded as a rare side effect of this filler. A 50-year-old female patient complained of swelling, dull pain, and heat in the right upper nasolabial fold area, which had started one week before her visit to Kyungpook National University Hospital. The patient received topical steroid therapy at a local clinic, which was not effective. At the injection site, a hard nodule was palpated and erythema was observed with mild tenderness. Antibiotic treatment and subsequent incision and drainage did not result in complete cure of the facial swelling, and the facial swelling and pain persisted. Computed tomography showed a lesion approximately 1-cm in size without clear boundaries and relatively increased nodular thickening. Finally, a subdermal lesion was removed via an intraoral vestibular approach. The lesion was diagnosed as inflammatory granuloma by a permanent biopsy. The patient had healed at two months after the filler injection. Although the soft tissue filler is widely used for cosmetic purposes, there is potential for complication, such as the inflammatory granuloma should be considered before treatment.
Go to : 
Various materials have been developed as skin fillers to correct the wrinkles of the eyes, forehead, cheeks, and around the mouth especially in middle-aged women. Filler injection has several advantages; for one, its cosmetic effect is shown immediately, and the procedure is relatively simple and convenient to perform in outpatient clinics. In addition, it does not interfere with daily life, and immediate return is possible. Among these fillers, Artecoll (Artes Medical Inc., San Diego, CA, USA) is a permanent synthetic cosmetic filler substance for the correction of facial wrinkles1. Consisting of polymethyl methacrylate microspheres of 30-40 µm with smooth surface (25%) suspended in bovine collagen (75%) used as carrier gel2, Artecoll is one of the best materials used today for lip augmentation and ridge corrections3. Because of the smooth surface of the granules and the lack of electrical charge, each microsphere is immediately encapsulated with the patient's own collagen4. After 3 months, the atelocollagen is replaced by the body's own connective tissue, which has been stimulated by the microspheres (stimulating the fibroblasts)4. The indications for Artecoll use are facial folds, lip and philtrum and chin augmentation, malar augmentation, scar revision, and other subdermal defects. Artecoll should be implanted subdermally between the dermis and the subcutis fat. An injection that is too superficial will cause blanching, whereas an injection that is too deep will not correct the folds1,5.
As the practice of filler injection gained popularity, an increa sing number of patients have been visiting the hospital for acute side effects such as swelling, pain, erythema, ecchy mo sis, bleeding or for delayed side effects including migration of the filler, inappropriate selection of the injection site, allergic reaction to the filler, granulomatous reaction, scar, infection, and tissue necrosis, etc2-5. Note, however, that inflammatory granuloma formation is a rather rare complication after filler injection. This complication had been suggested to be a consequence of foreign body reaction. In this report, we present a patient with inflammatory granuloma a month after having Artecoll filler injection on the upper lip for cosmetic purposes.
Go to : 
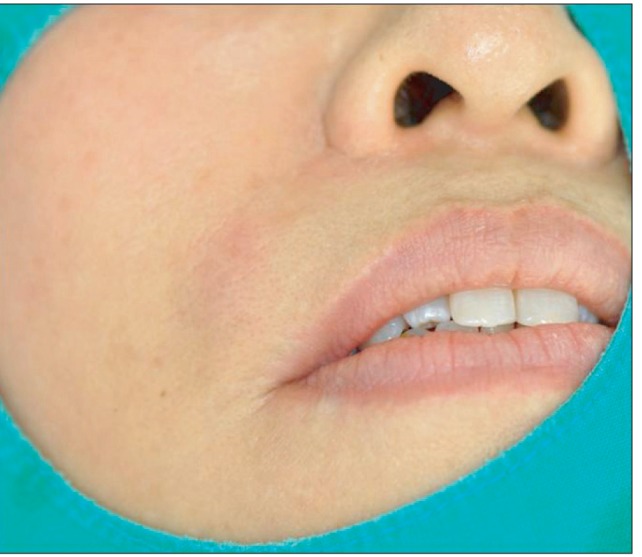
A 50-year-old healthy woman visited Kyungpook National University Hospital with chief complaint of intermittent dull pain on the right upper lip area. A month ago, she had soft tissue filler (Artecoll) injected into the upper lip at a local medical clinic. About twenty days later, the patient experienced swelling and dull pain in the right upper nasolabial fold area. Thus, steroid intralesional injection was performed in the local medical clinic. The symptoms did not subside, however. The patient visited our clinic with chief complaint of swelling, dull pain, and heat in the right upper nasolabial fold area. The skin examination revealed tenderness and hard nodules with slight overlying erythema on the right upper lip area.(Fig. 1) The patient was diagnosed with secondary infection of soft tissue filler. Incision and drainage combined with oral antibiotic therapy (Unasyn; Pfizer, New York, NY, USA) had been performed. Still, the symptom did not subside even after 3 days. Therefore, enhanced computed tomography (CT) was taken for accurate diagnosis.(Fig. 2)
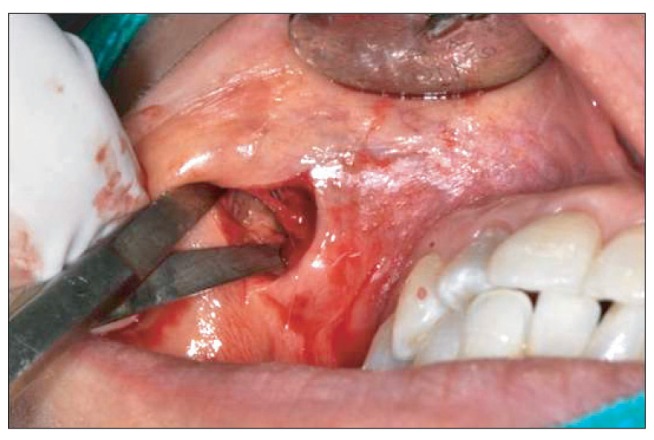
In the CT image, 1cm lesion without clear boundaries with enhanced nodular thickening was noted. Surgical excision under local anesthesia was performed (Fig. 3), followed by antibiotic therapy (Unasyn; three times a day, orally). A well-defined granulation mass in the subcutaneous tissue was removed by surgical excision. In the cause of smooth drainage, a drain was inserted for 3 days. For differential diagnosis, biopsy was performed. After a week, the symptom subsided.
The microscopic examination of H&E staining revealed epithelioid histiocytic granulomas with numerous multinucleated giant cells, with optically clear vacuoles as well as crystalloid materials.(Fig. 4)
Go to : 
The popularity of facial wrinkle correction via the injection of permanent biological inert implant materials is increasing. There are several biological and artificial materials such as bovine collagen, fibril, bioplastique, fluid silicone, gore-tex threads, and Artecoll6-8. Unfortunately, the durability of the correction with biological material lasts only for a very short time, and autodigest occurs within several months. Moreover, major adverse and allergic reactions have occurred occasionally after the injections of these implants1,7. Over the years, synthetic biomaterials have shown long durability, but complications have also been reported.
Before, Silicon and Gore-Tex were used as filler material. Nonetheless, irregular surface, accumulation of electrical charge, and high cost8 had been cited as disadvantages. Artecoll had been reported as better filler material compared to conventional fillers with its relatively bigger microparticles, zero electrical charge, and smoother surfaces with minimum heterogeneous substance since it undergoes a higher level of refinement process. It is also one of the popular fillers since it is not quickly removed from the tissue because its microparticles are not easily ingested by macrophages; neither do they migrate into the surrounding tissues9.
Granuloma formation against foreign material - one of the major side effects after filler injection - accounts for 3% of the cases, although it varies depending on the type of filler9. Artecoll has 0.01% foreign body reaction, which is very low compared to other materials, since it rarely stimulates the host's immune system owing to the previously described benefits10. The time taken for granuloma formation is generally 6-24 months after the injection, but a few cases had been reported after 10 years9,11. In this case, the foreign body granuloma formed a month after the injection, implying that the risk of granuloma formation exists even within a short period of time.
Granuloma formation rarely appears after filler injection, but its mechanism or natural progression has yet to be understood completely. Still, it had been reported with higher incidence when the injection was made on an improper site or was too shallow when the amount of filler was excessive or when the filler had low purity, particularly with 30% or more nanoparticles under 20 microns with rough surfaces. There has been a report of higher risk of granuloma formation when the filler is injected in a too shallow site, thereby making the skin blanch10. Our patient had received Artecoll injection in both upper lip areas, and the adverse response occurred only in the right upper lip. The biopsy result diagnosed the lesion as granuloma formation rather than infection. When viewed on CT, filler was injected too shallowly above the subcutaneous fat in the right upper lip area; thus inducing inflammatory granuoloma formation. A way to try to avoid this reaction is to inject Artecoll at the junction between the dermis and the subcutaneous fat4. Other risk factors related to granuloma formation are infection, psychological trauma, surgical maneuver, pregnancy, interferon treatment, etc., and these factors are considered to have certain effects on the host's immune system to induce abnormal foreign body reaction11-14. In this case, the patient had no particular medical history or risk factors related to granuloma formation; accordingly, she was regarded as a systematically healthy patient who may experience side effects related to filler injection. The histology reveals new fibroblast activity with thick bands of collagen fibers as in hypertrophic scarring dispersed with rare foreign body granulomas, i.e., numerous multinucleated giant cells containing optically clear vacuoles and crystalloid material3,7,15,16 as was found in our case.
Foreign body granuloma is most often treated with steroid injection into the lesion; other options are bleomycin injection into the lesion or topical imiquimod or 5-fluorouracil application. In addition, some reported a good outcome with oral steroid, minocycline, or allopurinol12,15,16. In this case, the steroid intralesional injection was not effective on the granuloma. Note, however, that the patient showed improvement after surgical excision of the lesion followed by drain insertion along with antibiotic therapy.
Artecoll filler is widely used for cosmetic purposes. Note, however, that potential complications such as inflammatory granuloma need to be monitored carefully after the injection. Various ways were also considered as treatment.
Go to : 
References
1. Lemperle G, Gauthier-Hazan N, Lemperle M. PMMA-Microspheres (Artecoll) for long-lasting correction of wrinkles: refinements and statistical results. Aesthetic Plast Surg. 1998; 22:356–365. PMID: 9767703.

2. Zimmermann US, Clerici TJ. The histological aspects of fillers complications. Semin Cutan Med Surg. 2004; 23:241–250. PMID: 15745233.

3. Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001; 23:197–202. PMID: 11391099.

4. Alcalay J, Alkalay R, Gat A, Yorav S. Late-onset granulomatous reaction to Artecoll. Dermatol Surg. 2003; 29:859–862. PMID: 12859389.

5. Requena C, Izquierdo MJ, Navarro M, Martínez A, Vilata JJ, Botella R, et al. Adverse reactions to injectable aesthetic microimplants. Am J Dermatopathol. 2001; 23:197–202. PMID: 11391099.

6. Engelman DE, Bloom B, Goldberg DJ. Dermal fillers: complications and informed consent. J Cosmet Laser Ther. 2005; 7:29–32. PMID: 16020214.

7. Lemperle G, Hazan-Gauthier N, Lemperle M. PMMA microspheres (Artecoll) for skin and soft-tissue augmentation. Part II: clinical investigations. Plast Reconstr Surg. 1995; 96:627–634. PMID: 7638287.
8. Hoffmann C, Schuller-Petrovic S, Soyer HP, Kerl H. Adverse reactions after cosmetic lip augmentation with permanent biologically inert implant materials. J Am Acad Dermatol. 1999; 40:100–102. PMID: 9922021.

9. Lemperle G, Romano JJ, Busso M. Soft tissue augmentation with artecoll: 10-year history, indications, techniques, and complications. Dermatol Surg. 2003; 29:573–587. PMID: 12786699.

10. Kim YJ, Park MY, Kim YC. Facial foreign body granuloma caused by filler (Artecoll) injection. Korean J Dermatol. 2008; 46:491–493.
11. Gelfer A, Carruthers A, Carruthers J, Jang F, Bernstein SC. The natural history of polymethylmethacrylate microspheres granulomas. Dermatol Surg. 2007; 33:614–620. PMID: 17451587.

12. Al-Qattan MM. Late artecoll granulomas aggravated by pregnancy. Ann Plast Surg. 2007; 58:592. PMID: 17452859.

13. Fischer J, Metzler G, Schaller M. Cosmetic permanent fillers for soft tissue augmentation: a new contraindication for interferon therapies. Arch Dermatol. 2007; 143:507–510. PMID: 17438184.

14. Lemperle G, Rullan PP, Gauthier-Hazan N. Avoiding and treating dermal filler complications. Plast Reconstr Surg. 2006; 118(3 Suppl):92S–107S. PMID: 16936549.

15. Conejo-Mir JS, Sanz Guirado S, Angel Muñoz M. Adverse granulomatous reaction to Artecoll treated by intralesional 5-fluorouracil and triamcinolone injections. Dermatol Surg. 2006; 32:1079–1081. PMID: 16918572.

16. McClelland M, Egbert B, Hanko V, Berg RA, DeLustro F. Evaluation of artecoll polymethylmethacrylate implant for soft-tissue augmentation: biocompatibility and chemical characterization. Plast Reconstr Surg. 1997; 100:1466–1474. PMID: 9385958.

Go to : 




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download