Abstract
Keratocystic odontogenic tumors (KCOT) - previously termed odontogenic keratocysts (OKC) - are characterized by aggressive behavior and a high rate of recurrence. Histopathologically, the basal layer of KCOT shows a higher cell proliferation rate and increased expression of anti-apoptosis genes. Clinically, KCOT is frequently involved in the mandibular posterior region but is not common in the posterior maxilla. However, it should be noted that due to its expansive characteristics, KCOT involved near the maxillary sinus could easily expand to an enormous size and occupy the entire maxilla. To achieve total excision of these expanded cystic tumors in the maxilla, a more aggressive approach would be needed. In this report, we describe two cases of expansile KCOT involving the entire unilateral maxilla and maxillary sinus; they were completely excised using the Weber-Ferguson approach, showing no evidence of recurrence during the follow-up period of more than two years. In immunohistochemical analyses of the tumor specimens, p53 and p63 showed strong expression, and B-cell lymphoma 2 (BCL2) and MKI67 (Ki-67) showed moderate or weak expression, however, detection of BCL2-associated X protein (BAX) was almost negative. These data indicate that expansile KCOT possesses increased anti-apoptotic activity and cell proliferation rate but decreased apoptosis. These properties of KCOT may contribute to tumor enlargement, aggressive behavior, and high recurrence rate.
Odontogenic keratocyst (OKC) was first introduced in the 1950s to describe keratin containing jaw cysts1. This cyst is considered an important odontogenic cyst because of its aggressive behavior, high recurrence rate, and specific histopathologic features, and it accounts for about 5-15% of all odontogenic cysts. In 2005, the World Health Organization reclassified OKC as "keratocystic odontogenic tumor" (KCOT)2. Recently, this tumor has been re-classified as a benign neoplasm of odontogenic origin and not as cyst; it is defined as a "benign uni- or multi-cystic odontogenic intraosseous tumor, with lining of parakeratinised stratified squamous epithelium and aggressive behavior"1-3. The cystic lumen is filled with creamy proteinaceous material or clear yellowish fluid, which is also an important diagnostic marker for KCOT1.
Several investigators agreed that KCOT arises from cell rests of dental lamina, and that its growth is related to enzymatic activity or unknown factors in the fibrous cystic wall4,5. Clinically, the posterior body of the mandible and ascending ramus especially related to impacted third molar were the prevalent occurring site of KCOT, showing slightly male predilection with peak incidence in patients between 10 and 40 years of age6,7. Radiologically, differently sized radiolucent lesions with displacement of impacted or erupted teeth, root resorption, or root displacement were easily detected in the jaw bones7. The treatment strongly recommended for KCOT consists of complete enucleation and curettage of the lesion to reduce the recurrence rate, although more conservative methods including marsupializa tion and decompression have also been tried in many cases. Interestingly, several recent studies observed the increased expressions of cell proliferation and anti-apoptosis related proteins such as MKI67 (Ki-67), tumor protein 53 (p53), tumor protein 63 (p63), B-cell lymphoma 2 (BCL2), and cyclooxygenase-2 (COX-2) in the basal cell layer of KCOT specimens, indicating that these proteins could be used as biological markers to diagnose KCOT5,8-14.
When odontogenic cysts or tumors including KCOT, calcifying odontogenic cyst, and dentigerous cyst are involved in the maxillary sinus, the cysts or tumors could asymptomatically expand and result in a large expanded cyst involving the whole maxilla and maxillary antrum15-19. To achieve total excision of expanded lesions and complete hemostasis in the anatomically delicate areas of the maxilla, a more extensive approach, such as Weber-Ferguson incision, would be needed. Here, we report two cases of large expanded maxillary KCOT, which occupied the entire maxilla and maxillary sinus. These expansile benign cystic tumors could be completely excised via wide facial approach through Weber-Ferguson incision. In addition, the tumor specimens were evaluated for the expression of BCL2, BCL2-associated X capital letter (BAX), Ki-67, and p53 and p63 proteins by immunohistochemistry to analyze their characteristics.
A 57-year-old man was referred to the clinic for the evaluation of facial swelling and asymmetry. The panoramic view showed a large cystic lesion from the anterior maxilla to the entire left side of the maxilla and maxillary sinus. The teeth involved showed no deviation, but slightly resorbed roots were detected. In the computed tomography (CT) views, the cystic tumor was expanded to the buccal and palatal bony wall with partial disruption of cortical bone.(Fig. 1) The lesion was tentatively diagnosed as KCOT because of its aggressive expansion with homogenous fluid-filled lumen. The patient underwent endodontic treatment for all teeth involved, and the expansile maxillary tumor was radically excised with Weber-Ferguson incision, showing no recurrent sign for more than 5 years' follow-up period.(Fig. 2)
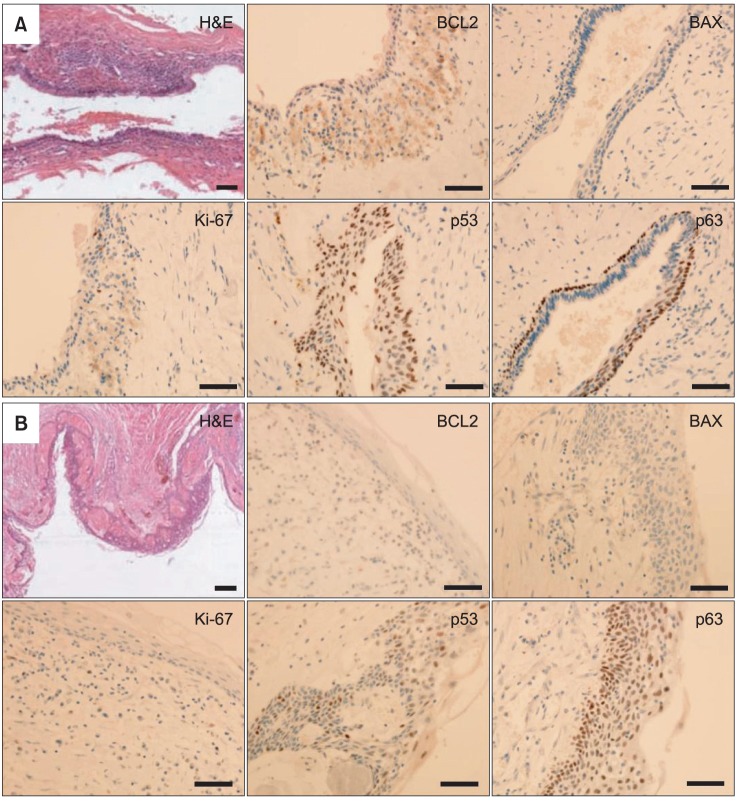
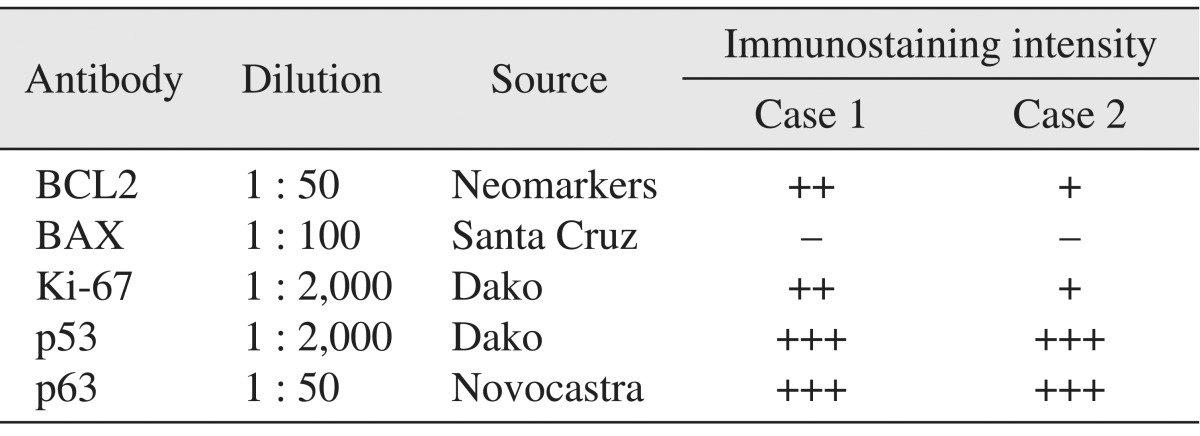
In the histopathological features of H&E-stained slides, thin lining of stratified squamous epithelium and thin parakeratotic surface on the lumen side of the cyst were observed, indicating KCOT. To characterize this tumor, specimens were immunostained with BCL2, BAX, Ki-67, and p53 and p63 antibodies. For the immunohistochemical analysis, tumor specimens were embedded in paraffin blocks, and then cut into 4-µm sections and mounted on silane-coated glass slides. Sections were maintained at room temperature for 12 hours, and then deparaffinized; after hydration they were immunostained using an automated immunostainer (BenchMark XT; Roche Korea, Seoul, Korea). The primary antibodies used and immunohistochemical staining results are summarized in Table 1. Positive immunostaining intensities were graded as +++, ++, +, and - for strong, moderate, weak, and negative staining, respectively.
A 54-year-old man visited for evaluation after sustaining unilateral facial swelling. Following the radiological examination, large expanded intrabony cystic lesion was found in the left maxilla and maxillary sinus. The lesion occupied the entire unilateral maxilla and maxillary sinus with the expansion and erosion of cortical bone. Tooth displacement was not detected, but some root resorption was observed in the teeth involved.(Fig. 3) Benign cystic lesion much like KCOT was diagnosed due to its radiological and clinical features and was completely excised with Weber-Ferguson incision. The patient had been followed up for 2 years without any evidence of recurrence.(Fig. 4)
The histopathological feature of H&E-stained slide was similar to that of case 1, with thin-lining epithelium and parakeratinized luminal surface as demonstrating features of KCOT. Immunohistochemical analysis of the tumor specimen was performed as described previously with case 1. Strong expression of p53 and p63, positive but weak detections of BCL2 and Ki-67, and negative detection of BAX were observed, and these expression patterns were similar to those of case 1.(Fig. 5. B, Table 1)
Multidirectional studies for KCOTs using various biomarkers of cell proliferation and apoptosis have been performed because KCOT is an aggressive benign neoplasm with high recurrence rate and extensive local invasion1. Higher proliferation rate and inhibition of apoptosis of cells are regarded as one of the most important steps in tumor formation, allowing the survival of genetically unstable cells and accumulating mutations that lead to neoplasm10,13. In previous studies, KCOT showed higher expression of cell proliferation markers particularly Ki-67, COX-2, proliferating cell nuclear antigen (PCNA) and p53, and epithelial stem cell factor p638-14. It also showed increased expression of BCL2, an anti-apoptosis marker, whereas the expression of BAX, a pro-apoptotic marker, in KCOT decreased13,20.
The p53 protein is expressed in the G1 phase of the cell cycle to allow repair of damaged DNA and to arrest cell cycle progression to the S phase. Some investigators reported that the overexpression of p53 in odontogenic tumors and cysts might be associated with increased cell proliferation12. The p63 protein has a role in epithelial development, stem cell biology, proliferation of limb and craniofacial-structures, and carcinogenesis. It may also have an important role in the progression of odontogenic cystic lesions because it is essentially of epithelial origin8,11. Ki-67 expression increased with cell cycle progression, reaching its peak during the G2 and M phases; thus suggesting that it can be used as cell proliferation marker12. In several studies, Ki-67 was expressed higher in the epithelium of KCOT than in the radicular cyst or oral mucosa, indicating that the epithelium of KCOT has higher cell proliferation rate9,10,12,13. Apoptosis plays an important role in the maintenance of cell homeostasis with programmed cell death for senescent or damaged cells. This mechanism has been regulated with pro-apoptosis and anti-apoptosis proteins. In previous studies, BCL2, one of the anti-apoptosis markers, showed higher expression level in the epithelium of KCOT specimens compared with radicular cyst13,20. Note, however, that BAX, the pro-apoptosis marker, was weakly expressed in KCOT13. These results demonstrate that the epithelium of KCOT has decreased apoptotic activity, allowing increased cell survival activity and invasive growth.
When odontogenic cysts or tumors formed in the maxilla, especially near or in the maxillary sinus, the lesion usually expanded to a large size15-19. For the complete excision of the expansile tumor and achievement of hemostasis in the maxilla particularly in anatomical fragile portions such as posterior maxillary wall and lateral nasal space and orbital floor, more extensive approaches are needed even though a lesion has no malignant evidences. Anatomically less dense structure, such as maxillary sinus, could allow the rapid growth of the lesion and tolerate the tumor's occupancy in the entire maxilla within a short period of time16,18,19. In this study, two cases of KCOT were developed and expanded in the unilateral entire maxilla and maxillary sinus, invading the opposite site of the maxilla. The anatomical structure, loose bone density of the maxilla, and empty space of the maxillary sinus could be considered one of the contributing factors for the development of expansile tumor in the present cases. Note, however, that the aggressive characteristics of KCOT in previous immunohistochemical studies enhanced cell proliferation rate and increased anti-apoptotic activity, possibly serving as other putative factors for enlarging tumor size.
In this report, the p53 and Ki-67 showed increased expre-ssion, although Ki-67 was weakly detected in the specimen in case 2, suggesting that the KCOTs of the present cases have higher cell proliferation rate. In addition, the anti-apoptotic factor, BCL2, showed moderate and weak expression in cases 1 and 2, whereas the pro-apoptotic factor, BAX, was almost negatively expressed in both cases. This result suggests that the present KCOT cases have decreased apoptosis activity and inhibit spontaneous cell death, leading to abnormal tumor growth. Moreover, the epithelial stem cell marker, p63 protein, showed high expression level in the basal layer and suprabasal layer of the tumor epithelium, indicating that KCOT is a tumor originating in epithelial tissue. Taken together, these properties of KCOT can contribute to tumor enlargement, aggressive behavior, and high recurrence rate.
References
1. Bhargava D, Deshpande A, Pogrel MA. Keratocystic odontogenic tumour (KCOT)--a cyst to a tumour. Oral Maxillofac Surg. 2012; 16:163–170. PMID: 22072419.

2. Barnes L, Eveson JW, Reichart P, Sidransky D. World Health Organization classification of tumors: pathology and genetics of head and neck tumours. Lyon: IARC Publishing Group;2005. p. 306–307.
3. Habibi A, Saghravanian N, Habibi M, Mellati E, Habibi M. Keratocystic odontogenic tumor: a 10-year retrospective study of 83 cases in an Iranian population. J Oral Sci. 2007; 49:229–235. PMID: 17928730.

4. Stoelinga PJ. Etiology and pathogenesis of keratocysts. Oral Maxillofac Surg Clin North Am. 2003; 15:317–324. PMID: 18088685.

5. Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002; 38:323–331. PMID: 12076694.
6. Eryilmaz T, Ozmen S, Findikcioglu K, Kandal S, Aral M. Odontogenic keratocyst: an unusual location and review of the literature. Ann Plast Surg. 2009; 62:210–212. PMID: 19158536.
7. Neville BW, Damn DD, Allen CM, Bouquout JE. Oral and maxillofacial pathology. 3rd ed. Philadelphia: Saunders;2009.
8. Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Fior A, et al. p63 expression in odontogenic cysts. Int J Oral Maxillofac Surg. 2005; 34:668–673. PMID: 16053892.

9. Gurgel CA, Ramos EA, Azevedo RA, Sarmento VA, da Silva Carvalho AM, dos Santos JN. Expression of Ki-67, p53 and p63 proteins in keratocyst odontogenic tumours: an immunohistochemical study. J Mol Histol. 2008; 39:311–316. PMID: 18256893.

10. Mendes RA, Carvalho JF, van der Waal I. A comparative immunohistochemical analysis of COX-2, p53, and Ki-67 expression in keratocystic odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111:333–339. PMID: 21215666.

11. Gonçalves CK, Fregnani ER, Leon JE, Silva-Sousa YT, Perez DE. Immunohistochemical expression of p63, epidermal growth factor receptor (EGFR) and notch-1 in radicular cysts, dentigerous cysts and keratocystic odontogenic tumors. Braz Dent J. 2012; 23:337–343. PMID: 23207846.

12. Gadbail AR, Patil R, Chaudhary M. Co-expression of Ki-67 and p53 protein in ameloblastoma and keratocystic odontogenic tumor. Acta Odontol Scand. 2012; 70:529–535. PMID: 21780975.

13. Soluk Tekkeşın M, Mutlu S, Olgaç V. Expressions of bax, bcl-2 and Ki-67 in odontogenic keratocysts (keratocystic odontogenic tumor) in comparison with ameloblastomas and radicular cysts. Turk Patoloji Derg. 2012; 28:49–55. PMID: 22207432.
14. de Oliveira MG, Lauxen Ida S, Chaves AC, Rados PV, Sant'Ana Filho M. Immunohistochemical analysis of the patterns of p53 and PCNA expression in odontogenic cystic lesions. Med Oral Patol Oral Cir Bucal. 2008; 13:E275–E280. PMID: 18449109.
15. Sikes JW Jr, Ghali GE, Troulis MJ. Expansile intraosseous lesion of the maxilla. J Oral Maxillofac Surg. 2000; 58:1395–1400. PMID: 11117688.

16. Cho JY, Nam KY. Expansile dentigerous cyst invading the entire maxillary sinus: a case report. J Korean Assoc Oral Maxillofac Surg. 2012; 38:245–248.

17. Kim SG, Park CY, Kang TH, Jang HS. Clinicopathologic study on cysts and postoperative cyst in maxillary sinus. J Korean Assoc Maxillofac Plast Reconstr Surg. 2000; 22:568–576.
18. Albright CR, Hennig GH. Large dentigerous cyst of the maxilla near the maxillary sinus: report of case. J Am Dent Assoc. 1971; 83:1112–1115. PMID: 5286148.

19. Altas E, Karasen RM, Yilmaz AB, Aktan B, Kocer I, Erman Z. A case of a large dentigerous cyst containing a canine tooth in the maxillary antrum leading to epiphora. J Laryngol Otol. 1997; 111:641–643. PMID: 9282204.

20. Diniz MG, Gomes CC, de Castro WH, Guimarães AL, De Paula AM, Amm H, et al. miR-15a/16-1 influences BCL2 expression in keratocystic odontogenic tumors. Cell Oncol (Dordr). 2012; 35:285–291. PMID: 22684875.

Fig. 1
Preoperative radiologic views of case 1. The panoramic view showed the large bone with the destroyed cystic lesion from the anterior maxilla to the entire left side of the maxilla and maxillary sinus. Partial dental root resorption of the posterior teeth involved was observed, but tooth deviation was not detected. In computed tomography view, homogenous cystic lesion was expanded to buccal and palatal bony wall with partial perforation of cortical bone.

Fig. 2
Intraoperative view and postoperative routine radiograms of case 1. The expansile maxillary cystic tumor was completely excised with Weber-Ferguson's approach, and all teeth involved were retained. Panorama and Water's view 1 year after surgery; there was no evidence of recurrence, showing normal sinus function.

Fig. 3
Preoperative panorama and computed tomography views of case 2. Large cystic lesion with cortical bone expansion on the entire left side of the maxilla and maxillary sinus was detected. Partial root resorption of the teeth involved was observed similar to case 1.
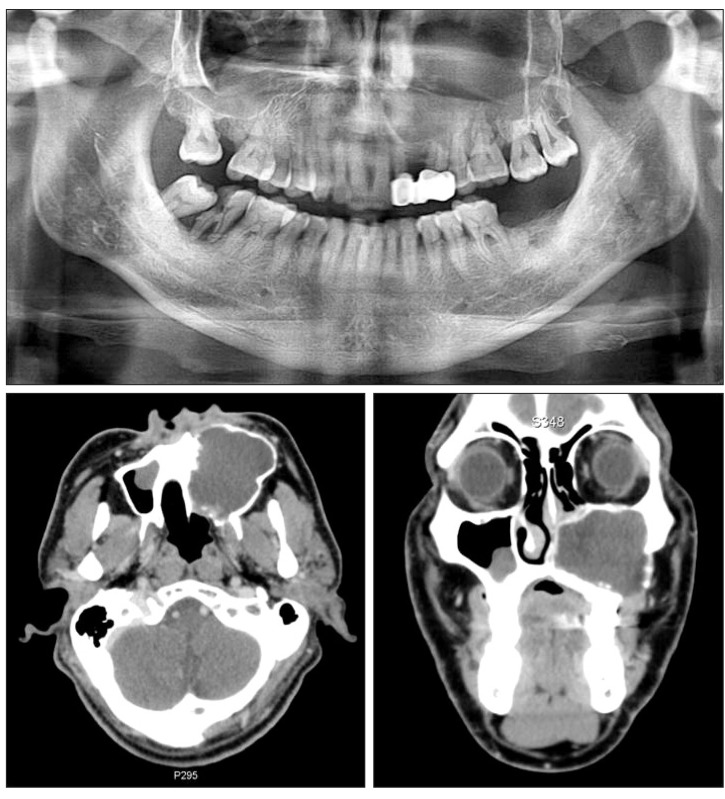
Fig. 4
Intraoperative views, tumor specimen, and postoperative radiograms and clinical view of case 2. The expansile maxillary cystic tumor was completely excised via Weber-Ferguson's approach, and there was no clinical evidence of any recurrence or complication for follow-up period.

Fig. 5
Histological and immunohistochemical (IHC) analysis of tumor specimens of case 1 (A) and case 2 (B). In H&E-stained slides of two cases, thin lining epithelium and parakeratin formation in the lumen side of the tumors were observed, indicating that the tumor was KCOT. In the IHC analysis of the specimens, MKI67 (Ki-67) was moderately expressed in case 1 and weakly reacted in case 2; tumor protein 53 (p53) and tumor protein 63 (p63) were strongly expressed in the basal and suprabasal layers of the tumor epithelium in two cases. B-cell lymphoma 2 (BCL2) was moderately expressed in the stromal and epithelial layers of tumors, whereas BCL2-associated X protein (BAX) was almost negatively detected in the specimens in two cases. Scale bar=100 µm.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download