Abstract
Objectives
This study investigated the question of whether adenoviral magnetofection can be a suitable method for increasing the efficacy of gene delivery into bone marrow stromal cell (BMSC) and for generation of a high level of bone morphogenic protein (BMP) secretion at a minimized viral titer.
Materials and Methods
Primary BMSCs were isolated from C57BL6 mice and transduced with adenoviral vectors encoding β galactosidase or BMP2 and BMP7. The level of BMP secretion, activity of osteoblast differentiation, and cell viability of magnetofection were measured and compared with those of the control group.
Results
The expression level of β galactosidase showed that the cell transduction efficiency of AdLacZ increased according to the increased amount of magnetic nanoparticles. No change in cell viability was observed after magnetofection with 2 µL of magnetic nanoparticle. Secretion of BMP2 or BMP7 was accelerated after transduction of AdBMP2 and 7 with magnetofection. AdBMP2 adenoviral magnetofection resulted in up to 7.2-fold higher secretion of BMP2, compared with conventional AdBMP2-transduced BMSCs. Magnetofection also induced a dramatic increase in secretion of BMP7 by up to 10-fold compared to the control. Use of only 1 multiplicity of infection (moi) of magnetofection with adenoviral transduction of AdBMP2 or AdBMP7 resulted in significantly higher transgene expression compared to 20 moi of conventional adenoviral transduction.
Stromal cells isolated from fresh bone marrow aspirates have multilineage potential that can differentiate into a variety of cell populations1. Note, however, that the optimization of gene delivery to bone marrow stromal cells (BMSCs) from mammalian cells had limited efficiencies.
Recently, gene delivery by polyethylenimine-conjugated supraparamagnetic nano-particle under a magnetic field - so-called magnetofection - was introduced. Scherer et al.2 and Plank et al.3 first reported that the application of magnetic nanoparticles to gene vectors to various cells showed significantly enhanced uptake of these vectors and subsequent high target protein expression. The magnetic force applied on gene vector-magnetic particle complex can increase the accumulation of these complexes on the surface of the various cells. In particular, cell lines that have only limited efficiencies with regard to target gene expression, such as human endothelial cells, can be successfully managed by magnetofection4-6. Note, however, that there had been few attempts to apply magnetofection to enhanced gene delivery for BMSC.
Bone morphogenic protein 2 (BMP2) gene transfer by transfection of BMP2-cDNA to BMSC can be differentiated into osteoprogenitor cells, affecting the neighboring cells by paracrine pathway to potentiate the regeneration of various types of bone defects7,8. BMP7 was also reported as a potent osteogenic mediator8. To accelerate gene expression, various gene delivery systems with high transfection efficiency had been proposed9,10. Although there had been advances in gene transfer using non-viral vectors, efficiency of gene transfer is reported to be approximately 10-9 compared to adenoviral vectors8. Since enhanced transfection efficiency is key to investigating the function of gene and protein, the viral gene transfer method had been proposed as a good method for gene overexpression. Different BMP2 gene therapy approaches to bone regeneration have been studied, and viral vectors such as adenoviruses11-13 and retroviruses14 as carriers of BMP2 cDNA had been used. In particular, adenoviruses had been regarded as a most efficient gene transfer vehicle15. Nonetheless, immunogenicity of viral capsid protein is a major problem; exposure of cell to virus for a long period of time or at higher doses is toxic for several tissues and cells; hence the need for a method of potentiating viral transduction to the target cell at low multiplicity of infection (moi) to minimize the side effects16.
This study sought to investigate whether adenoviral magnetofection can be a suitable method for increasing the efficacy of gene delivery into BMSC. As far as we know, this is the first trial of osteogenic induction of BMSC using adenoviral magnetofection.
Primary mouse BMSC were isolated from the tibia and femur of 8-10-week-old C57BL6 mice. Mice were sacrificed by cervical dislocation, both ends of the bilateral tibia and femur were snipped off, and bone marrow was flushed out with Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (FBS) and antibiotics (penicillin 100 U/mL, streptomycin 100 µg/µL). The single cell suspension of bone marrow was collected by several repetitions of drawing the cells into a syringe. The cells were seeded on a 100 mm dish, and non-adherent cells were removed by changing the media (DMEM with 10% FBS) for three consecutive days. These cells of the first passage culture were then re-plated prior to confluence. The culture media was replaced every 2 days and cultured up to 4 weeks. For the experiments of the study, the cells at passage 3 were used for in vitro viral transduction and in vivo animal experiments.
The E1-deleted adenoviral vector containing human BMP2 or BMP7 cDNA - which constitutively expresses BMP2 or BMP7, respectively, under the control of the CMV promoter - was propagated in 293A cells. Viral lysates were then expanded and purified using the Adeno-X virus purification kit (Clontech, Mountain View, CA, USA) and titrated with the Adeno-X Rapid titer kit (Clontech). The final viral stock titer was 2×109 pfu/mL on 293A cells.
BMSCs were plated in 24-well plates (in vitro study) or 10 cm culture dish (in vivo study) at a density of 35,000 cells/cm2 on the day before transduction. For the in vitro adenoviral transduction of BMSC, adenovirus (AdBMP-2, -7, AdLacZ) at the desired titer was added to cells in 500 µL of serum-free DMEM and incubated for 6 hours. The virus-containing medium was replaced by complete culture medium. After 24 hours, the media were then changed to DMEM with 10% FBS and ascorbic acid (50 µg/mL) and β-glycerol phosphate (5 mM) to enhance osteoblastic differentiation and were replaced every two days.
For adenoviral transduction with magnetofection, AdBMP-2, -7 and LacZ were diluted with DMEM to establish the desired titer and mixed with 2 µL of magnetic particle (Magnetofectin, CombiMac; Chemicell GmbH, Berlin, Germany) indicated otherwise. The mixture was incubated for 20 minutes at room temperature and added dropwise to the cells. The cell culture plate was placed upon a magnetic plate (MagnetoFACTOR-24 plate; Chemicell GmbH) for 30 minutes. The virus/magnetic particle-containing medium was replaced by complete culture medium. The media were then replaced in the same manner as adenoviral transduction.
Two days after transduction, the expression of β galactosidase (number of blue nuclei/field) according to the varying amount of magnetic nanoparticles and viral particles was evaluated with X-gal staining kit (OZ Bioscience, Marseille, France) based on the manufacturer protocol. The dependence on moi at different magnetic nanoparticles 48 hours after magnetofection was also assessed. The efficiency of gene transfer with adenoviral magnetofection was compared with that of conventional adenoviral transduction.
BMP2 and 7 proteins secreted into conditioned media at the indicated time points were measured after adenoviral transduction or adenoviral magnetofection with AdBMP-2, 7 or LacZ, using commercially available ELISA kit (Quantikine; R&D Systems, Minneapolis, MN, USA). The conditioned media were prepared by culturing cells in serum-free medium for 24 hours.
Alkaline phosphatase (ALP) activity measured in cell layers was evaluated with p-nitrophenyl phosphate substrate included in a commercially available kit (Quanti -Chrom ALP assay kit; BioAssay Systems, Hayward, CA, USA). The sample was harvested in a lysis buffer containing 0.1% NP 40 and homogenized through three cycles of -70℃ freezing and 36℃ thawing. ALP activity was measured according to the manufacturer's instruction and expressed in terms of relative luminescence unit (RLU)/mg protein. Protein content was measured with protein assay kit (BioRad, Hercules, CA, USA.) using bovine serum albumin. For ALP staining, cells were cultured for 8 days in mineralizing medium, and then fixed for 30 seconds in 4% formaldehyde, washed three times with PBS, and stained with ALP kit (Sigma, St. Louis, MO, USA).
To compare the relative toxicity caused by magne-tofection, 3-(4,5-Dimethylthiazol-2-yl)-2.5-dipheny-ltetrazoliumbromide (MTT) assay was utilized. BMSCs were seeded into the 96-well plate (1×104 cells/well/100 µL media), and magnetic particle was added 48 hours after seeding. After 72 hours of incubation, 50 µL of MTT (Cell proliferation kit I; Roche Molecular Biochemicals, Mannheim, Germany) was added to each well. After another 4 hours of incubation at 37℃, the supernatant of the plate was removed, and 150 µL dimethylsulfoxide (DMSO) was added to the cell monolayer. Absorbance was measured at 520 nm (Model 680; Bio-Rad). The statistical difference between groups was analyzed with ANOVA using SPSS Program (Version 10; SPSS Inc., Chicago, IL, USA).
The staining result showed that β galactosidase expression increased significantly with the increased viral titer of AdLacZ, and that there were definitively more staining positive cells in the magnetofection group.(Fig. 1. A) When the efficiency of AdLacZ transduction was calculated by stained cells per microscopic field, magnetofection revealed at least 400 times' higher efficiency compared to conventional adenoviral transduction.(Fig. 1. B) The efficiency of the AdLacZ transdu-ction with 200 moi was dependent on the increased amount of magnetic particles (from 1 to 9 µL of magnetic nanoparticles). There was no significant difference in the shape or viability of BMSC according to the increased amount of magnetic particles when applied to AdLacZ transduction.(Fig. 2)
The time course of BMP2 or 7 secretion after conventional adenoviral transduction vs. adenoviral magnetofection was investigated. AdBMP2 20 moi showed significantly (2.8 fold) higher BMP2 secretion compared to BMSC. Only 1 moi of AdBMP2, a suboptimal level for BMP2 induction, did not enhance BMP2 secretion. Note, however, that magnetofection with 1 moi of AdBMP2 induced 7.2-fold higher BMP2 protein secretion compared to BMSC control or 1 moi of the AdBMP2 transduction group. This difference was highest at 3 days, slowly decreasing over time until 7 days after the transduction.(Fig. 3)
The BMP7 protein secretion in conditioned media of BMSC was examined using a method similar to BMP2 ELISA. At day 3, BMP7 was secreted by 6.7-fold when 1 moi of AdBMP7 was transduced with magnetofection; on the other hand, 20 moi of conventional AdBMP7 increased secretion by 5.2-fold compared to the control (all P<0.05). Such magnetofection dramatically increased BMP7 secretion by 10-fold compared to the control until 7 days of the transduction. Despite the decrease in cell viability over time after the magnetofection, the resultant BMP-2 or 7 expression remained at a high level until 7 days after the transduction with magnetofection in both AdBMP2 and 7.(Fig. 4)
To measure the ALP activity, BMSCs were harvested 1, 3, and 7 days after transduction. Values were expressed as fold changes relative to untreated BMSC cells compared to the ALP activity on the 1st day. On day 7 of transudation, AdBMP2 adenoviral magnetofection showed 2.4-fold and 1.8-fold higher ALP activity than non-transduced BMSC and conventional AdBMP2 (1 moi)-transduced BMSC.(Fig. 5. A)
BMSCs transduced with the respective adenoviral vectors were cultured with osteogenic medium containing β-glycerol phosphate and ascorbic acid and stained. Increased mineralization activity was noted at the ALP staining in adenoviral magnetofection compared to the control.(Fig. 5. B)
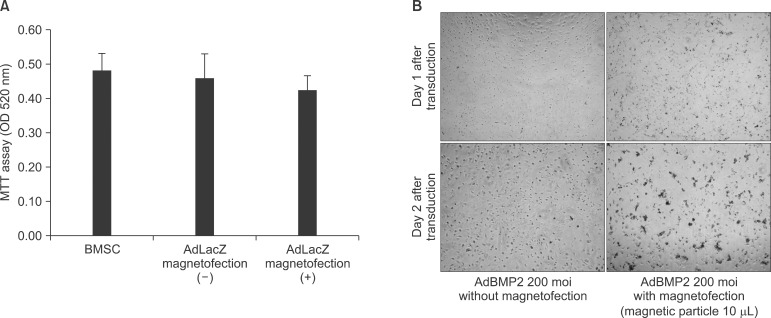
Cell viability was accessed through the comparison of relative toxicity caused by magnetofection. Cytotoxicity was ob served when high dose (10 µL) of magnetic nanoparticle was applied with AdBMP2 virus.(Fig. 6. A) This was not the case with the AdLacZ viral transduction given high dose of ma gnetic nanoparticle as shown in Fig. 2. The use of 2 µL of magnetic nanoparticle for AdLacZ viral transduction did not show significantly increased cell apoptosis compared to conventional adenoviral transduction (P>0.05).(Fig. 6. B)
Acceleration of the osteogenic induction of BMSC can be achieved by the transduction of the cells to express proteins that enable differentiation of BMSC lineage to osteoblast. Even though BMPs had been widely used for bone regeneration in various clinical and experimental settings, problems of short half-life and difficulty in continuous protein delivery system remain17. Moreover, large and single amount of BMP2 or 7 administrations can be accumulated in organs that were not targeted at the time of administration; this can result in unwanted bone formation in kidneys or liver. Therefore, another system needs to be considered for continuous gene delivery. For this purpose, the adenoviral gene delivery system has a definitive advantage in ex vivo or in vivo gene therapy since the adenovirus does not integrate into the host genomic DNA; there is also a low likelihood of malignant transformation of host cells18.
Two of the most promising candidates are BMP2 and BMP7. Adenoviruses containing BMP2 or 7 cDNA under the control of aCMV promoter AdBMP2 and 7 can reportedly be used for gene therapy for bone regeneration19. This study also proved the efficiency of BMP2 and 7 adenoviral gene transfer. Note, however, that a serious side effect from the host immune reaction against the clinical application of adenovirus had been reported previously20-22. Moreover, there is limitation in the application of suboptimal dose of adenoviral transduction if the target cell surface is deficient in Coxakie-adenovirus receptor (CAR)23. High efficiency (>90%) of adenovirus transduction and consistent level of CAR expression in human marrow stromal cells24 as well as definitively higher gene expression level compared to conventional liposomal transfection had been reported. The transduction efficiency of BMSC and other mesenchymal cells is lower compared to other cell lines. For example, a previous study reported that AdLacZ at 250-500 pfu/cell showed transduction efficiency of 30-40%17.
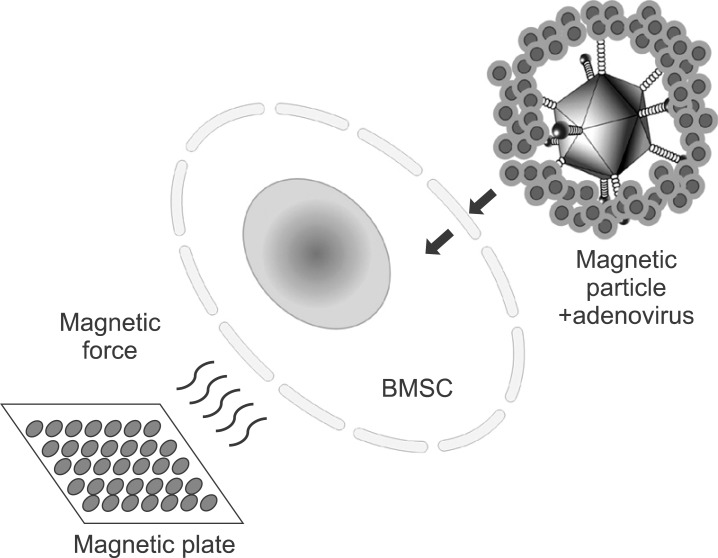
Magnetic particle-associated adenoviral gene delivery had been introduced to enhance the efficiency of gene expression but minimize the associated risk by reducing the viral dose25. According to Krötz et al.6, the increased effectiveness of magnetofection could be attributed to an increased concentration of pDNA or viral particle/magnetic complexes at the outer cellular membrane. Adenovirus solutions are mixed with magnetic nanoparticle and usually incubated for 20 minutes at room temperature. The negatively charged electrokinetic viral particles in aqueous media assemble with cationic polymer such as polyethyleneimine, a major component of the outer coating of magnetic nanoparticle core26. This cationic lipid-encapsulated adenovirus particle triggers accelerated binding to cell membranes.(Fig. 7) When the magnetic field is applied to magnetic nanoparticle-adenovirus complex, the transduction efficiency dramatically increased as also shown in our experiments. Definitive adenoviral transduction can reportedly be performed even on cells without CAR, which does not allow the penetration of adenovirus27.
The results of this study showed no significant cell apoptosis even with high amount (10 µL) of magnetic nanoparticles with AdLacZ. Similarly, human umbilical vein endothelial cells showed that, although the 10 µL of magnetic particle treatment showed increased cytotoxicity compared to 1 µL, viability was maintained at above 85% level at optimal viral titer28. When high adenoviral titer of AdBMP2 (200 moi) and high dose of magnetic particles were applied, however, cell apoptosis was observed under the microscope. Magnetic particles of 2 µL did not induce significant cell apoptosis in the AdBMP2 adenoviral transduction. This suggests that optimizing the transduction efficiency with relative low cytotoxicity would be possible, but it would differ according to the type of adenoviral gene. Therefore, the optimal value of magnetic nanoparticles for mouse BMSC primary culture cells needs to be investigated based on the type of gene.
Our result showed that adenoviral magnetofection induced continuously high gene expression until 6 days after the transduction of AdBMP2 or 7. BMP2 secretion was dramatically accelerated by up to 7.2-fold compared to non-transduced BMSC or minimal dose (1 moi) of conventional AdBMP2-transduced BMSC. AdBMP7 adenoviral magnetofection also showed dramatically increased BMP7 secretion by up to 10-fold compared to the control until 7 days of the transduction. Clearly, a minimal amount of viral particles can be potentiated by the magnetic nanoparticle-induced gene expression system. This result implies that magnetofection can be used for the optimal system for gene therapy for bone regeneration.
The magnetic nanoparticle-induced transduction or transfection is now widely accepted even for induced pluripotent stem (iPS) cells resembling embryonic stem cells29. Since the iPS cell needs strong expression of the four defined transcription factors - Oct3/4, Klf4, Sox2, and c-Myc-viral vector was the method of choice in a previous report30. Now, magnetofection can be one of the methods of choice that circumvent the disadvantage of viral transduction31. A recent animal experiment had shown targeted vascular endothelial growth factor (VEGF) gene delivery by using magnetic nanoparticle-adenoviral vectors and revealed enhanced cardiac regeneration in the targeted region29. Since the isolation of the magnetic cell separation was also applied to in vitro and in vivo settings, magnetofection can be a safe and promising strategy for gene therapy27 that can be applied to bone regeneration. Note, however, that potentiated protein secretion with viral vector at much lower dose of viral particles does not directly mean zero toxicity and immunologic response in humans. Further investigation is needed for the clinical application of this method.
The results of this study showed that magnetic particle-mediated gene transudation is a highly efficient method of gene delivery to BMSC, outweighing the problem of toxicity of magnetofection. These results also suggest that magnetofection can reduce the effective minimal amount of AdBMPs for bone regeneration.
The magnetic particle-mediated gene transduction is a highly efficient method of gene delivery to BMSC. Magnetofection can lower the amount of viral particles (MOI) while improving efficacy of gene delivery. This advantageous effect can be useful in preventing adenovirus inactivation and minimizing immune response in gene therapy.
References
1. Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001; 19:180–192. PMID: 11359943.

2. Scherer F, Anton M, Schillinger U, Henke J, Bergemann C, Krüger A, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002; 9:102–109. PMID: 11857068.

3. Plank C, Scherer F, Schillinger U, Bergemann C, Anton M. Magnetofection: enhancing and targeting gene delivery with superparamagnetic nanoparticles and magnetic fields. J Liposome Res. 2003; 13:29–32. PMID: 12725725.

4. Edgell CJ, Curiel DT, Hu PC, Marr HS. Efficient gene transfer to human endothelial cells using DNA complexed to adenovirus particles. Biotechniques. 1998; 25:264–268. 270–272. PMID: 9714887.

5. Tanner FC, Carr DP, Nabel GJ, Nabel EG. Transfection of human endothelial cells. Cardiovasc Res. 1997; 35:522–528. PMID: 9415297.

6. Krötz F, de Wit C, Sohn HY, Zahler S, Gloe T, Pohl U, et al. Magnetofection--a highly efficient tool for antisense oligonucleotide delivery in vitro and in vivo. Mol Ther. 2003; 7:700–710. PMID: 12718913.

7. Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, et al. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cell-mediated gene therapy. J Gene Med. 1999; 1:121–133. PMID: 10738576.

8. Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000; 78:476–486. PMID: 10861845.

9. Gottschalk S, Sparrow JT, Hauer J, Mims MP, Leland FE, Woo SL, et al. A novel DNA-peptide complex for efficient gene transfer and expression in mammalian cells. Gene Ther. 1996; 3:448–457. PMID: 9156807.
10. Haines AM, Irvine AS, Mountain A, Charlesworth J, Farrow NA, Husain RD, et al. CL22 - a novel cationic peptide for efficient transfection of mammalian cells. Gene Ther. 2001; 8:99–110. PMID: 11313779.

11. Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, et al. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999; 14:1290–1301. PMID: 10457261.

12. Olmsted EA, Blum JS, Rill D, Yotnda P, Gugala Z, Lindsey RW, et al. Adenovirus-mediated BMP2 expression in human bone marrow stromal cells. J Cell Biochem. 2001; 82:11–21. PMID: 11400159.

13. Lieberman JR, Daluiski A, Stevenson S, Wu L, McAllister P, Lee YP, et al. The effect of regional gene therapy with bone morphogenetic protein-2-producing bone-marrow cells on the repair of segmental femoral defects in rats. J Bone Joint Surg Am. 1999; 81:905–917. PMID: 10428121.

14. Breitbart AS, Grande DA, Mason JM, Barcia M, James T, Grant RT. Gene-enhanced tissue engineering: applications for bone healing using cultured periosteal cells transduced retrovirally with the BMP-7 gene. Ann Plast Surg. 1999; 42:488–495. PMID: 10340856.
15. Verma IM, Somia N. Gene therapy-promises, problems and prospects. Nature. 1997; 389:239–242. PMID: 9305836.
16. Partridge K, Yang X, Clarke NM, Okubo Y, Bessho K, Sebald W, et al. Adenoviral BMP-2 gene transfer in mesenchymal stem cells: in vitro and in vivo bone formation on biodegradable polymer scaffolds. Biochem Biophys Res Commun. 2002; 292:144–152. PMID: 11890685.

17. Franceschi RT, Yang S, Rutherford RB, Krebsbach PH, Zhao M, Wang D. Gene therapy approaches for bone regeneration. Cells Tissues Organs. 2004; 176:95–108. PMID: 14745239.

18. Russell WC. Update on adenovirus and its vectors. J Gen Virol. 2000; 81:2573–2604. PMID: 11038369.

19. Zhao M, Zhao Z, Koh JT, Jin T, Franceschi RT. Combinatorial gene therapy for bone regeneration: cooperative interactions between adenovirus vectors expressing bone morphogenetic proteins 2, 4, and 7. J Cell Biochem. 2005; 95:1–16. PMID: 15759283.

20. Raper SE, Chirmule N, Lee FS, Wivel NA, Bagg A, Gao GP, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003; 80:148–158. PMID: 14567964.

21. Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther. 2002; 9:979–986. PMID: 12522437.

22. Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999; 286:2244–2245. PMID: 10636774.

23. Bergelson JM. Receptors mediating adenovirus attachment and internalization. Biochem Pharmacol. 1999; 57:975–979. PMID: 10796067.

24. Hung SC, Lu CY, Shyue SK, Liu HC, Ho LL. Lineage diffe-rentiation-associated loss of adenoviral susceptibility and Coxsackie-adenovirus receptor expression in human mesenchymal stem cells. Stem Cells. 2004; 22:1321–1329. PMID: 15579649.

25. Sapet C, Laurent N, de Chevigny A, Le Gourrierec L, Bertosio E, Zelphati O, et al. High transfection efficiency of neural stem cells with magnetofection. Biotechniques. 2011; 50:187–189. PMID: 21486240.

26. Sapet C, Pellegrino C, Laurent N, Sicard F, Zelphati O. Magnetic nanoparticles enhance adenovirus transduction in vitro and in vivo. Pharm Res. 2012; 29:1203–1218. PMID: 22146803.

27. Veiseh O, Gunn JW, Zhang M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv Drug Deliv Rev. 2010; 62:284–304. PMID: 19909778.

28. Plank C, Vlaskou D, Schillinger U, Mykhaylyk O. MagnetofectionTM platform: from magnetic nanoparticles to novel nucleic acid therapeutics. Ther Deliv. 2011; 2:717–726. PMID: 22822504.
29. Zhang Y, Li W, Ou L, Wang W, Delyagina E, Lux C, et al. Targeted delivery of human VEGF gene via complexes of magnetic nanoparticle-adenoviral vectors enhanced cardiac regeneration. PLoS One. 2012; 7:e39490. PMID: 22844395.

30. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007; 131:861–872. PMID: 18035408.

31. Park HY, Noh EH, Chung HM, Kang MJ, Kim EY, Park SP. Efficient generation of virus-free iPS cells using liposomal magnetofection. PLoS One. 2012; 7:e45812. PMID: 23049868.

Fig. 1
Efficiency of gene transfer with adenoviral magnetofection compared to conventional adenoviral transduction. The evaluation of expression of β galactosidase with X-gal staining involved counting the number of blue nuclei/field. β galactosidase expression increased significantly according to the use of magnetofection (A) and increased viral titer of adLacZ (B). Magnetofection (2 µL of magnetic particle) revealed at least 400 times' higher efficiency compared to conventional adenoviral transduction. (×100)
(moi: multiplicity of infection)
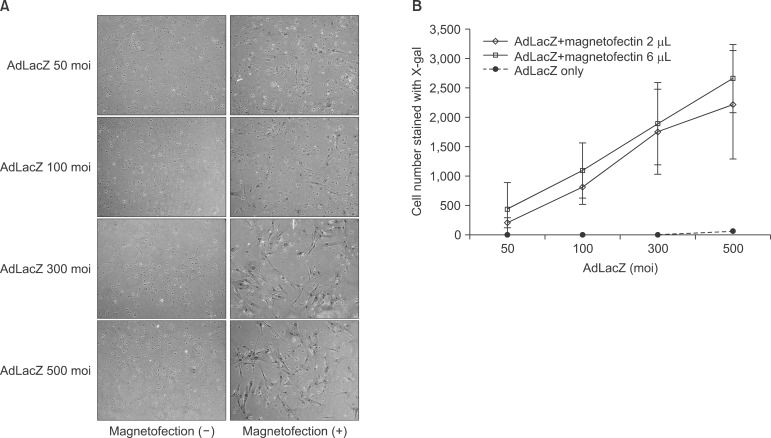
Fig. 2
Dependence on varying magnetofectin (magnetic nanoparticle) dose at constant multiplicity of infection (moi) (AdLacZ 200 moi). The efficiency of the transduction was potentiated according to the increased amount of magnetic particles without significant cell apoptosis. (×100)
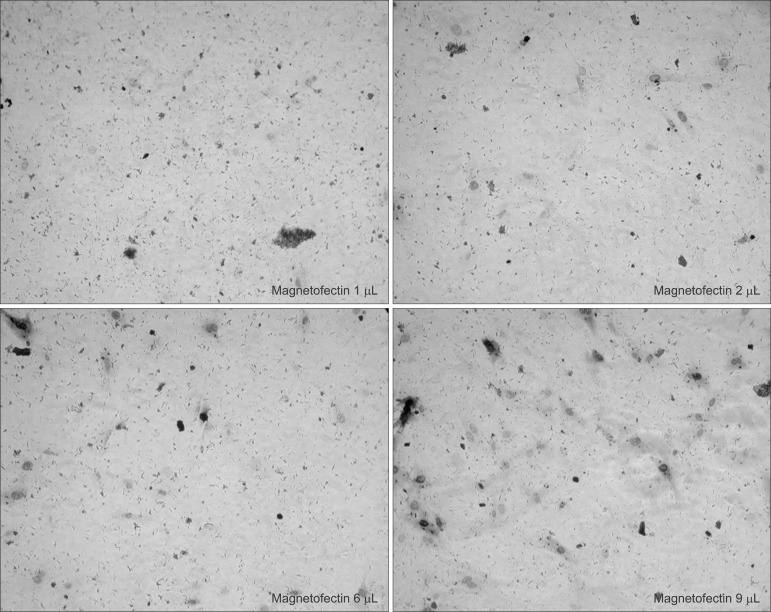
Fig. 3
BMP2 protein in conditioned media of bone marrow stromal cell (BMSC) transduced with AdBMP2 with/without magnetic nanoparticle. BMSCs were transduced with the indicated individual AdBMP2 titer. The conditioned media were prepared by culturing cells in serum-free medium for 24 hours. Levels of BMP2 were measured using specific ELISA kits.
(moi: multiplicity of infection).
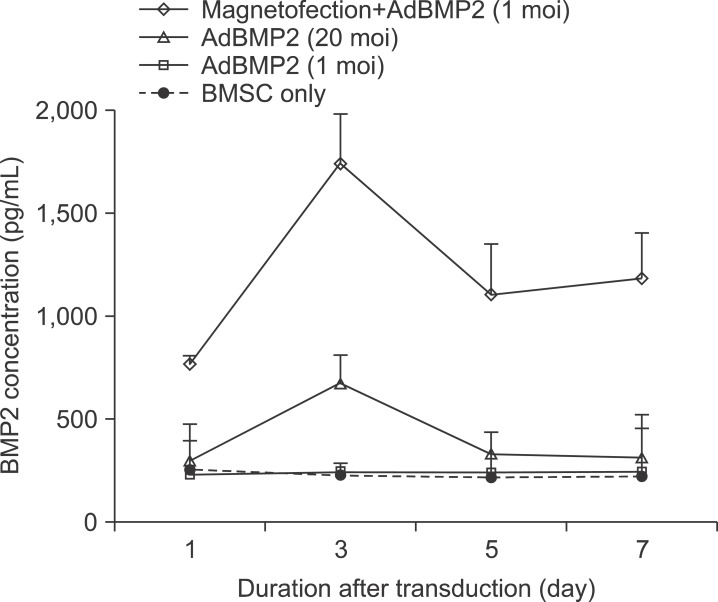
Fig. 4
BMP7 protein in conditioned media of bone marrow stromal cell (BMSC) transduced with AdBMP7 with/without magnetic nanoparticle. BMSCs were transduced with the indicated individual AdBMP7 titer. The conditioned media were prepared and measured using a method similar to BMP2. The application of magnetofection accelerated BMP7 secretion from BMSC.
(moi: multiplicity of infection).
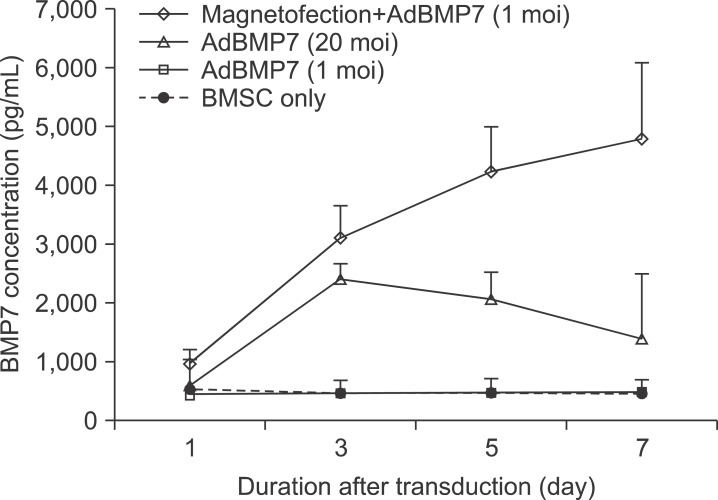
Fig. 5
Alkaline phosphatase (ALP) activity of transduced bone marrow stromal cell (BMSC) with different multiplicity of infection (moi) or inclusion of magnetofection (A) and assayed for mineralization by ALP staining after culturing for 8 days in mineralizing medium (B).
(Mag: magnetofection)
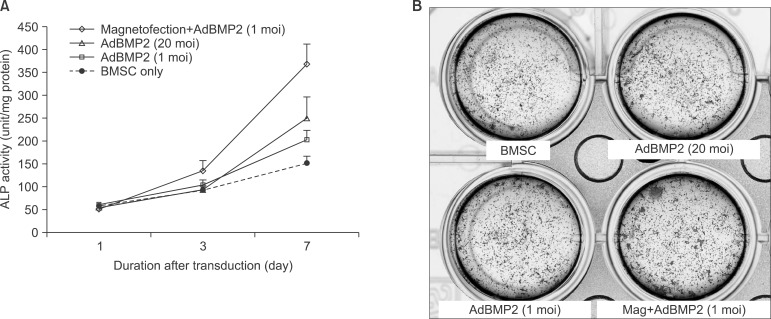
Fig. 6
Evaluation of cell viability. (A) Magnetofection with 2 µL of magnetic particle in adenoviral gene transfer did not change cell viability significantly (P>0.05). The comparison of the relative toxicity caused by magnetofection was evaluated with 3-(4,5-Dimethylthiazol-2-yl)-2.5-diphenyltetra-zoliumbromide (MTT) assay. (B) Magnetofection with high amount (10 µL) of magnetic particles and high dose of AdBMP2 (200 moi) triggered cell apoptosis. (×100)
(OD: optical density, moi: multiplicity of infection, BMSC: bone marrow stromal cell, BMP: bone morphogenic protein)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download