Abstract
Objectives
Odontogenic keratocysts (OKCs) have a tendency to recur and possess an aggressive nature. the aim of the present study was to evaluate cytokeratin (CK) expression patterns as a method for the differentiation between dentigerous cysts (DCs) and OKCs, as their histomorphologic appearance are often indistinguishable.
Materials and Methods
Formalin-fixed, paraffin-embedded tissue sections of 43 OKCs and 38 DCs were immunohistochemically analyzed with i-solution in a quantitative manner in order to evaluate the immunoreactivity of CK 10, 16 and 17.
Results
CK 10 expression was evident in 79.1% of OKCs but found in only 18.4% of DCs (P<0.05), and CK 10 expression was observed to occur more frequently in OKCs (mean 25.45%) than in DCs (2.19%) (P<0.05). The expression of CK 16 was evident in 79.1% of OKCs but found in only 7.9% of the DCs (P<0.05) and CK 16 expression was observed to occur more frequently in OKCs (mean 4.33%) than in the DCs (0.61%) (P<0.05). The expression of CK 17 was evident in 88.4% of OKCs but seen in only 15.7% of the DCs (P<0.05) and CK 17 expression was observed to occur more frequently in OKCs (mean 31.11%) than in the DCs (2.37%) (P<0.05).
Odontogenic keratocysts (OKCs) of the jaw are developmental odontogenic cysts arising from cell rests of dental lamina. First identified and described by Philipsen1 in 1956, Pindborg and Hansen2 suggested the histologic criteria necessary to diagnose OKCs in 1963 as follows: OKCs have very thin epithelial lining, even thickness, and rete ridge formation is inconspicuous. The basal epithelial layer is composed of a palisaded layer of cuboidal or columnar epithelial cells. The luminal surface shows corrugated appearance and thin fibrous wall is commonly devoid of any inflammatory infiltration. OKCs are divided into general parakeratinized type and rare orthokeratinized type, with the former reported to have the aforesaid features3.
OKCs tend to invade surrounding tissues aggressively and recur frequently owing to their thin, fragile cystic wall, small satellite cyst within the fibrous wall, osteolytic materials generated on the cystic wall, and continuous proliferation of epithelium. Due to these histological and clinical features, not only histopathological but also genetic and immunohistochemical studies have been conducted1,4-6.
Among the existing genetic and immunohistochemical studies, Toller7 in 1967 suggested that OKCs be regarded as benign cystic neoplasm rather than cysts considering various clinical features such as fast invasion and destruction of surrounding tissues and high recurrence rate. Likewise, Shear, Chen et al., Stoll et al. suggested on "Is It a Benign Cystic Neoplasm?"(2000) that OKCs be regarded as a benign tumor based on the existing genetic and immunohistochemical studies6,8-11. Based on these research, World Health Organization recommended in 2005 regarding OKC as a benign tumor and named it keratocystic odontogenic tumor12.
Immunohistochemical research on this field detects specific substances using antigen-antibody reaction that the antibody binds to that specific antigen only. This is an effective method of achieving the desired results by analyzing the sensitivity of a part to various antigens from a specimen13.
With the development of immunohistochemistry, the type of soft tissue tumor was divided more specifically based on the subtle distinction of their differentiation. Note, however, that such histochemical method is an additional tool for histomorphologic evaluation (microscopic examination), not a substitution14.
Recently, many research studies on the differentiation and proliferation of odontogenic cyst have been conducted using an immunohistochemical method for cytokeratin (CK)9,11,15,16, Ki-676, PCNA6, p53 protein6, and epidermal growth factor9.
In particular, cytokeratin is a protein with molecular weight of 40-67 kD, is located in epithelial cells, and is composed of polypeptide. There are 20 types of cytokeratin, and their expression and distribution are determined by the type and layer of intraoral epithelial cell. Generally, cytokeratin is regarded as the best indicator of epithelial differentiation; the comparison of expression of cytokeratins between OKCs and other odontogenic cysts or soft tissue tumor did not always yield the same results11,15-21.
Therefore, this study was conducted to evaluate the expression of CK 10, 16 and 17 in OKCs and dentigerous cysts, provide information for immunohistochemical research on the cytokeratin of OKCs, and evaluate whether cytokeratins can be a useful indicator for the differential diagnosis of dentigerous cysts.
We immunohistochemically examined 43 cases of 38 patients diagnosed with OKCs based on the biopsy (21 were male and 17 were female; average age of 29.9) and 38 cases of 35 patients diagnosed with dentigerous cysts (18 were male and 17 were female; average age of 20.4) who visited the Department of Oral and Maxillofacial Surgery, Kyungpook National University Dental Hospital from January 1, 2008 to December 31, 2009. Among the 38 OKC patients, 3 patients had OKCs in 2 areas, whereas 1 patient had it in 3 places. On the other hand, among the 35 dentigerous cyst patients, 1 patient had it in 4 places.
For immunohistochemical stain, the biopsy tissues of patients with OKCs or dentigerous cysts were processed as follows: they were fixed with 10% neutral formalin, dehydrated, and embedded in paraffin; after removing the paraffin, to block endogenous peroxidase, they were soaked in 0.3% peroxide, washed with water, and microwaved for 10 minutes with pH 6.0 citrate buffer to improve the exposure of tissue antigen. To block a non-specific combination between antigen and antibody, they were treated with blocking reagent (UltraTech HRP; Immunotech, Marseille, France) for 7 minutes prior to the reaction with the primary antibody and then reacted with 3 primary antibodies (Cytokeratin 10, 16 and 17; Novocastra, Newcastle, United Kingdom), respectively. Afterward, the secondary antibody with biotin attached was processed with peroxidase reagent with avidin attached. The color was developed with diaminobenzidine and the sections were counterstained with hematoxylin. Based on the data above, to measure the expression of CK 10, 16 and 17 in the epithelium of OKCs and dentigerous cysts, we used i-solution (IMT i-Solution Inc., Vancouver, Canada)- a phase analysis program that calculates the accurate component ratio by dividing substances in the image based on the difference in brightness and color- and applied the Wilcoxon rank sum test because the result did not show normal distribution.
CK 10 was observed in 34 (79.1%) out of 43 cases of OKC and in 7 (18.4%) out of 38 cases of dentigerous cyst.(Table 1)
Among 43 cases of OKC, the mean expression of CK 10 was 25.45%, and standard deviation (SD) was 89.75. Among 38 cases of dentigerous cyst, mean expression and SD were 2.19% and 6.60, respectively. A higher expression of CK 10 was observed in the OKC group, and the difference was statistically significant.(Table 2, Figs. 1, 2)
CK 16 was observed in 34 (79.1%) out of 43 cases of OKC and in 3 (7.9%) out of 38 cases of dentigerous cyst.(Table 3)
Among 43 cases of OKC, the mean expression of CK 16 was 4.33%, and SD was 7.65. Among 38 cases of dentigerous cyst, mean expression and SD were 0.61% and 2.80, respectively. A higher expression of CK 16 was observed in the OKC group, and the difference was statistically significant.(Table 4, Figs. 3, 4)
CK 17 was observed in 38 (88.4%) out of 43 cases of OKC and in 6 (15.7%) out of 38 cases of dentigerous cyst.(Table 5)
Among 43 cases of OKC, the mean expression of CK 17 was 31.11%, and SD was 22.43. Among 38 cases of dentigerous cyst, mean expression and SD were 2.37% and 7.47, respectively. A higher expression of CK 17 was observed in the OKC group, and the difference was statistically significant.(Table 6, Figs. 5, 6)
Recently, research studies on dentigerous cysts, radicular cysts, conventional OKCs, and OKCs related to nevoid basal cell carcinoma syndrome using cytokeratin, Ki-67, PCNA, and p53 have been conducted. Compared to dentigerous cysts and radicular cysts, they are more evident in OKCs and are expressed much more obviously and frequently in OKCs related to a syndrome6.
In particular, cytokeratin is an intermediate filament protein (IFP) located in epithelial cells. IFPs cohere and settle in cytoplasm with microfilaments and micro-tubes in the shape of parallel skein with diameter of 7-10 nm and consist biochemically and functionally of keratin, vimentin, desmin, neurofilament protein, glial fibrillary acidic protein, and lamin. In epithelial cells, IFPs have massive keratin and, more importantly, express a different distribution pattern in tumor cell and non-tumor cell. Various genes of multiple chromosomes participate in the formation of IFPs. In the case of keratin, it is formed by genes in chromosomes 12q and 17q. A higher number of cytokeratin means lower molecular weight. The acidic type (Type 1) has 12 cytokeratins (CK9-20), whereas the basic type (Type 2) has 8 cytokeratins (CK 1-8). Most cytokeratins express a pair of acidic-type and basic-type keratins in the development of cells14. The cytokeratin pattern and distribution differ by type and layer of intraoral epithelial cells.
Generally, cytokeratin is regarded as the best indicator of epithelial differentiation. The purpose of the study on the expression of keratin in OKC is to evaluate its usefulness as an indicator in the differential diagnosis of odontogenic cyst such as radicular cysts, dentigerous cysts, and OKCs, to identify the cause of cyst by comparing the expression of cytokeratin in the oral mucosa and odontogenic epithelium of the developmental cyst, and to identify the mechanism of aggressiveness of OKCs22.
OKCs are distinguised from ameloblastoma and dentigerous cyst. Both dentigerous cysts and OKCs are arised from the odontogenic epithelium, and they have similar radiological characteristics. Nonetheless, they should be distinguished for treatment because dentigerous cysts hardly recur after proper enucleation or marsupialization22-24, whereas OKCs show high recurrence rate25.
CK 10 is an acidic-type cytokeratin generated in the epidermis and keratinized squamous epithelium with CK 1. According to Lessin et al.26 CK 10 is an intermediate-sized, acidic-type CK generated in the suprabasal cell of a differentiated epidermis with CK 1, not in the basal cell. In the immunohistochemical research to distinguish OKCs and dentigerous cysts using CK, August et al.17 reported that CK 10 was observed only in OKCs in all samples, not in dentigerous cysts or radicular cysts. Therefore, CK 10 can be an indicator to distinguish OKC and 2 other cysts. In the research of Matthews et al.27 in the case of epithelium of keratinized cysts, CK 10 was observed only in 4 out of 32 cases, whereas OKCs were observed in 17 out of 18 cases. Similarly, Stoll et al.11 used CK 10 in the differential diagnosis of dentigerous cyst, radicular cyst, and OKC and in immunohistochemical analysis; as a result, it was observed in 3 out of 30 cases of radicular cysts and in 9 out of 30 cases of dentigerous cysts in the superficial layer but was not observed in other layers. CK 10 was observed in 7 out of 15 cases of OKCs and in all cases for the suprabasal cell layer. Therefore, CK 10 can be regarded as an important factor that is generated more in OKC than radicular cyst. In other research studies using monoclonal antibodies LH2 and LH3, CK 10 was not observed in OKCs18. In 1987, Hormia et al.19 reported that CK 10 was not observed in OKCs but was observed in 2 out of 18 cases of dentigerous cysts because a different type of antibody was used for cytokeratin. Note, however, that some research studies showed different results even when the same antibody (LH3, LH2) was used for CK 10 in the epithelial tissue of OKCs18-20,28. In this research, CK 10 was observed in the suprabasal layer, with much higher frequency and intensity of expression in OKCs than dentigerous cysts.
CK 16 is an acidic-type cytokeratin generated in the squamous cell with CK 6. According to Smith and Matthews28 CK 16 reacts strongly in the epithelial layer of OKCs due to the active proliferation of OKCs. In 1989, Gao et al.18 reported a similar result to our study, i.e., CK 16 was very evident in OKCs. In other research studies, CK 16 was observed in dentigerous cysts and radicular cysts9. Moreover, in 1998 and 1991, Matthews and colleagues27,28 investigated the expression of keratin 13 and 16- which share the same epitopes- using a specific antibody to keratin 13; as a result, CK 16 was observed in all OKCs, dentigerous cysts, and radicular cysts. Similar to CK 10, however, tests of CK 16 did not always show the same results. In this research, the frequency and intensity of CK 16's expression in the suprabasal layer were much higher in OKCs.
CK 17 is a cytokeratin generated in basal cells and myoepithelial cells with CK 727. According to Stoll et al.11 CK 17 was observed more in OKCs than in other cysts when distinguishing dentigerous cysts, radicular cysts, and OKCs using immunohistochemical analysis. CK 17 was observed in 14 out of 15 cases of OKC, in 12 out of 30 cases of radicular cyst, and in 9 out of 30 cases of dentigerous cyst11. Meara et al.15 also reported that CK 17 was evident in the epithelial layer of all OKCs for patients with nevoid basal cell carcinoma syndrome, but not in dentigerous cysts. Therefore, CK 17 can be a useful indicator in distinguishing OKC and other cysts. In 1987, however, Hormia et al.19 reported that CK 17 was observed in both OKC and dentigerous cyst. In this research, the frequency and intensity of CK 17's expression were much higher in OKC than in dentigerous cyst. In particular, CK 17 was more evident than CK 10 and 16.
As shown in this research and other research studies, in the immunohistochemical research of cytokeratin, the interpretation of results should be carried out more carefully because the masking of all or part of the epitopes may occur in any cell, and the same keratin may yield different results according to the type of monoclonal antibody.
The expression of CK 10, 16 and 17 was higher in OKCs than in dentigerous cysts. Such result of this study is a theoretical basis of the OKC's clinical features such as aggressiveness and high recurrence rate. Moreover, it can be a valuable additional parameter distinguishing OKCs and dentigerous cysts when distinguishing is difficult, and it can also be useful in determining clinical treatment methods. Note, however, that immunoblotting should be used in combination to evaluate the expression of cytokeratin by such diseases more correctly29-31, and monoclonal antibodies should be developed to distinguish antigens in a more current manner.
In 43 cases of 38 patients diagnosed with OKCs and 38 cases of 35 patients diagnosed with dentigerous cysts visiting the Department of Oral and Maxillofacial Surgery, Kyungpook National University Dental Hospital from January 1, 2008 to December 31, 2009, the expression of CK 10, 16 and 17 was observed highly in OKCs compared to dentigerous cysts.
Therefore, we think that the expression of cytokeratin may reflect the high recurrence and aggressive nature of OKCs, and that the expression of cytokeratin can be a valuable additional parameter distinguishing between dentigerous cysts and OKCs as well as a useful clinical treatment.
Figures and Tables
Fig. 1
Photomicrograph of the epithelium of odontogenic keratocyst. The maximum intensity of the immunohistochemical expression of cytokeratin (CK) 10 and basal and suprabasal cells were strongly positive for CK 10 (H&E staining, ×40).
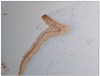
Fig. 2
Photomicrograph of the epithelium of the dentigerous cyst. The mean intensity of the immunohistochemical expression of cytokeratin (CK) 10 and basal cells were weakly positive for CK 10 (H&E staining, ×40).

Fig. 3
Photomicrograph of the epithelium of odontogenic keratocyst. The maximum intensity of the immunohistochemical expression of cytokeratin (CK) 16 and basal and suprabasal cells were strongly positive for CK 16 (H&E staining, ×40).
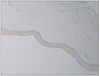
Fig. 4
Photomicrograph of the epithelium of the dentigerous cyst. The mean intensity of the immunohistochemical expression of cytokeratin (CK) 16 and basal cells were weakly positive for CK 16 (H&E staining, ×40).
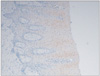
Fig. 5
Photomicrograph of the epithelium of odontogenic keratocyst. The maximum intensity of the immunohistochemical expression of cytokeratin (CK) 17 and basal and suprabasal cells were strongly positive for CK 17 (H&E staining, ×40).
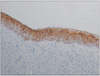
Fig. 6
Photomicrograph of the epithelium of the dentigerous cyst. The mean intensity of the immunohistochemical expression of cytokeratin (CK) 17 and basal cells were almost negative for CK 17 (H&E staining, ×40).

References
1. Philipsen HP. Om keratocyster (kolesteatomer) i kaeberne. Tandlaegebladet. 1956. 60:963–980.
2. Pindborg JJ, Hansen J. Studies on odontogenic cyst epithelium: 2. Clinical and roentgenologic aspects of odontogenic keratocysts. Acta Pathol Microbiol Scand. 1963. 58:283–294.
3. Barnes L. Surgical pathology of the head and neck. 1986. 1st ed. New York: Marcel Dekker Inc..
4. el-Hajj G, Anneroth G. Odontogenic keratocysts--a retrospective clinical and histologic study. Int J Oral Maxillofac Surg. 1996. 25:124–129.

5. Chow HT. Odontogenic keratocyst: a clinical experience in Singapore. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998. 86:573–577.
6. Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 2. Proliferation and genetic studies. Oral Oncol. 2002. 38:323–331.
7. Toller P. Origin and growth of cysts of the jaws. Ann R Coll Surg Engl. 1967. 40:306–336.
8. Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 1. Clinical and early experimental evidence of aggressive behaviour. Oral Oncol. 2002. 38:219–226.

9. Shear M. The aggressive nature of the odontogenic keratocyst: is it a benign cystic neoplasm? Part 3. Immunocytochemistry of cytokeratin and other epithelial cell markers. Oral Oncol. 2002. 38:407–415.

10. Li TJ, Kitano M, Chen XM, Itoh T, Kawashima K, Sugihara K, et al. Orthokeratinized odontogenic cyst: a clinicopathological and immunocytochemical study of 15 cases. Histopathology. 1998. 32:242–251.

11. Stoll C, Stollenwerk C, Riediger D, Mittermayer C, Alfer J. Cytokeratin expression patterns for distinction of odontogenic keratocysts from dentigerous and radicular cysts. J Oral Pathol Med. 2005. 34:558–564.

12. Philipsen HP. Barnes L, Eveson JW, Reichart P, Sidransky D, editors. Keratocystic odontogenic tumor. World Health Organization classification of tumors: pathology and genetics of head and neck tumors. 2005. Lyon: IARC Press;306–307.
13. Taylor CR, Shi S-R, Barr NJ, Wu N. Dabbs DJ, editor. Techniques of immunohistochemistry. Diagnostic immunohistochemistry. 2002. Philadelphia, Pennsylvania: Churchill Livingstone;1–44.

14. Cerilli LA, Wick MR. Dabbs DJ, editor. Immunohistology of soft tissue and osseous neoplasm. Diagnostic immunohistochemistry. 2002. Philadelphia, Pennsylvania: Churchill Livingstone;59–112.
15. Meara JG, Pilch BZ, Shah SS, Cunningham MJ. Cytokeratin expression in the odontogenic keratocyst. J Oral Maxillofac Surg. 2000. 58:862–865.

16. MacDonald AW, Fletcher A. Expression of cytokeratin in the epithelium of dentigerous cysts and odontogenic keratocysts: an aid to diagnosis. J Clin Pathol. 1989. 42:736–739.

17. August M, Faquin WC, Troulis M, Kaban LB. Differentiation of odontogenic keratocysts from nonkeratinizing cysts by use of fine-needle aspiration biopsy and cytokeratin-10 staining. J Oral Maxillofac Surg. 2000. 58:935–940.

18. Gao Z, Mackenzie IC, Cruchley AT, Williams DM, Leigh I, Lane EB. Cytokeratin expression of the odontogenic epithelia in dental follicles and developmental cysts. J Oral Pathol Med. 1989. 18:63–67.

19. Hormia M, Ylipaavalniemi P, Nagle RB, Virtanen I. Expression of cytokeratins in odontogenic jaw cysts: monoclonal antibodies reveal distinct variation between different cyst types. J Oral Pathol. 1987. 16:338–346.

20. Morgan PR, Shirlaw PJ, Johnson NW, Leigh IM, Lane EB. Potential applications of anti-keratin antibodies in oral diagnosis. J Oral Pathol. 1987. 16:212–222.

21. Gao Z, Mackenzie IC, Williams DM, Cruchley AT, Leigh I, Lane EB. Patterns of keratin-expression in rests of Malassez and periapical lesions. J Oral Pathol. 1988. 17:178–185.

22. Ahlfors E, Larsson A, Sjögren S. The odontogenic keratocyst: a benign cystic tumor? J Oral Maxillofac Surg. 1984. 42:10–19.

23. Vedtofte P, Praetorius F. Recurrence of the odontogenic keratocyst in relation to clinical and histological features. A 20-year follow-up study of 72 patients. Int J Oral Surg. 1979. 8:412–420.

24. Zachariades N, Papanicolaou S, Triantafyllou D. Odontogenic keratocysts: review of the literature and report of sixteen cases. J Oral Maxillofac Surg. 1985. 43:177–182.

25. Neville BW, Damm DD, Allen CM, Bouquot JE. Neville BW, Damm DD, Allen CM, Bouquot JE, editors. Odontogenic cysts and tumors. Oral & Maxillofacial Pathology. 1995. Philadelphia: W.B. Saunders Company;497–500.

26. Lessin SR, Huebner K, Isobe M, Croce CM, Steinert PM. Chromosomal mapping of human keratin genes: evidence of non-linkage. J Invest Dermatol. 1988. 91:572–578.

27. Matthews JB, Mason GI, Browne RM. Epithelial cell markers and proliferating cells in odontogenic jaw cysts. J Pathol. 1988. 156:283–290.

28. Smith AJ, Matthews JB. Browne RM, editor. Odontogenic epitheliunm and its residues. Investigative pathology of the odontogenic cysts. 1991. Boca Raton: CRC Press;53–85.
29. Thesleff I, Ekblom P. Distribution of keratin and laminin in ameloblastoma. Comparison with developing tooth and epidermoid carcinoma. J Oral Pathol. 1984. 13:85–96.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download