Abstract
Basal cell adenoma (BCA) of the parotid gland is a rare benign tumor. In the parotid gland, BCA is occasionally difficult distinguish from adenoid cystic carcinoma in terms of clinical and pathological perspectives. An adenoid cystic carcinoma of the parotid gland grows slowly but spreads persistently to the surrounding tissues, particularly along the perineural spaces. In the present case, BCA of the parotid gland was misdiagnosed as an adenoid cystic carcinoma. We discuss the reason for such a misdiagnosis, and present a method for making a correct diagnosis.
Basal cell adenoma (BCA) of the salivary glands is a rare benign neoplasm having a monomorphous histological appearance dominated by basaloid cells1. The reported data state the incidence of BCA in all salivary neoplasms to be 1-3%2,3. It appears most frequently in the parotid gland in adults1,4. Clinically, it is generally a slow-growing, asymptomatic, freely movable mass1,4.
As one of the most common and best recognized malignant salivary tumors5, adenoid cystic carcinoma (ACC) was first reported in 1853 by Robin, Lorain, and Laboulbene6. ACC was originally called cylindroma because of its histopathological morphology. ACC was recorded to be located in the major and minor salivary glands; usually small with an incomplete capsule, it has a propensity toward perineural spread5. It has high, almost inevitable predisposition to recur in a person with old age, occurring even when radical excision has been performed7.
Clinically and histopathologically, there are similar features between BCA and ACC. The treatment plan is completely changed by the result of diagnosis, especially when malignant or benign; hence the need for different diagnoses. In this paper, we report BCA misdiagnosed as ACC in the parotid gland, review literature, and discuss the different diagnoses of similar cases.
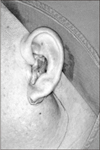
In December 2010, a 50-year-old man was referred to our hospital for evaluation of a palpable mass in the left parotid region. He complained of severe burning and pulling sensation in the left pre-auricular area. The painful sensation began about 2 years ago, continuing intermittently but not worsening. The physical examination revealed a mass measuring about 5×4 cm, which was hard, tender, and movable.(Fig. 1) There was no symptom on facial nerve function and cervical lymphadenopathy. The magnetic resonance image showed a well-defined, non-homogeneously enhanced mass on the deep portion of the left parotid gland. (Fig. 2) The T1W1 image revealed moderate to low signal intensity (Fig. 2. A), but the T2W1 image showed higher signal intensity portion in the middle and medial site of the lesion suspected to be a necrotic lesion.(Fig. 2. B) There was no evidence of infiltrating margin.
Partial parotidectomy was performed. We approached the mass by pre-auricular incision.(Fig. 3. A) The superficial parotid layer of the parotid gland was removed, and the mass was excised.(Fig. 3. B) The facial nerve trunk was conserved. (Fig. 3. C)
Macroscopically, an encapsulated whitish lesion measuring 4×4×2 cm was observed. The microscopic examination showed infiltrating epithelial strands with multiple cystic changes and solid pattern. The tumor cells rarely showed keratinization and consisted almost exclusively of intermediate cell type. The tumor cells were small and cuboidal, exhibiting deeply basophilic nuclei and little cytoplasm; mitotic activity was rarely seen. There was no perineural invasion (Fig. 4), however. Immunohistochemical examination was done as well. The inmmunostain of proliferating cell nuclear antigen was frequently positive in tumor cells, but that of p53 was hardly visible. The immunostain of cytokeratin-7 was frequently positive in glandular structure, but the same cannot be said for cytokeratin-14. The immune stain of snail was frequently positive in tumor cells, but that of beta-catenin was hardly visible. Pan-K and S-100 were positively stained, but wnt-1 was negative.(Fig. 5) Initially, the pathologist considered the lesion to be ACC.
Nonetheless, we had doubts on the pathological diagnosis because it was not in accord with the patient's clinical symptoms and radiological feature. Thus, we requested a reexamination on the section to another clinical pathologist. The final diagnosis was BCA, not ACC. The cells of the islands were palisaded and cuboidal in shape, with the trabecular subtype demonstrating narrow, cordlike epithelial strands. There was no evidence of malignancy.
There was slight facial weakness in one third of the lower left part of the face. The symptom persisted for 6 months but eased gradually. The patient was monitored for 1 year, and there was no evidence of the tumor or signs and symptoms recurring.
BCA in the salivary gland is a rare benign neoplasm, consisting of isomorphic basaloid tumor cells4,8. Constituting only 1% of all salivary neoplasms2,9, it is controversial for its gender predominance8. The tumor can grow at any age but is most common among old-age adults8. BCA occurs in epithelial cells, usually in the terminal duct8. Histologically, it has many variants such as solid, tubular, trabecular, and membranous10. The most common variant is the solid type, but each tumor has combination-type variants10. BCA consists of 2 types of cells10: one is a small cell with insufficient cytoplasm and round-shaped nucleus10, and the other type has large eosinophilic cytoplasm and ovoid-shaped nuclei10.
There are some benign and malignant neoplasms that must be differentially diagnosed with BCA. Pleomorphic adenoma is clinically similar to BCA. It appears as a slowly growing, freely movable mass1, typically appearing as a painless, firm mass10. Pleomorphic adenoma of the parotid gland mostly occurs in the superficial lobe, manifesting swelling on the preauricular area10. Accounting for 53-77% of parotid tumors10, pleomorphic adenoma originated with a mixture of ductal and myoepithelial elements10. In contrast, the basic tumor pattern is very variable, but the individual cells are rarely pleomorphic10. When we evaluated the patient clinically, we easily supposed that the lesion to be pleomorphic adenoma because it is the most common benign tumor in the parotid gland.
ACC accounts for 10% of all tumors in salivary glands5,11. The parotid gland area is the most common site in the head and neck5. Histopathologically, ACC can be classified into three morphological patterns including cribriform, tubular, and solid5. The most important and unique feature of ACC is the tendency of perineural invasion, even in early-stage tumors5,10. In most cases, the cytological typing of ACC is distinguished by the detection of large globules of extracellular matrix surrounding the basaloid tumor cells5. ACC shows the most histological similarities to BCA, since both have the same developmental origin10. Note, however, that their characters are very much different in terms of the integrity of the basal layer, number of mitoses, and growth speed. In the parotid gland, ACC is rare, accounting for only 2-3% of all tumors10,11. There is fairly equal gender distribution, although some studies have shown slight female predilection10.
In our case, the lesion was growing slowly, and it was separated with adjacent normal tissue. The mass was firm, painless, and freely movable. The patient did not complain about his lesion until 2 years ago. He had no facial palsy and lymphadenopathy. Therefore, clinically, it had a benign character, so we believed it to be a benign tumor. Nonetheless, the histopathological result was ACC, although we could not believe the result. We requested for the ex ami nation of the sample to another pathologist, expecting a different result. True enough, it was BCA; we accepted the result because of many clinical similar evidences.
It is difficult to diagnose basal cell adenoma in the parotid gland, since it is uncommon in the parotid gland and it seems to be just another tumor. The surgeon must consider the patient's clinical symptom, radiological symptom, and pathological symptom.
Figures and Tables
Fig. 2
A. In T1W1 magnetic resonance imaging, there is a well-defined and non-homogeneously enhanced mass on the deep portion of left parotid gland. B. There is higher signal intensity portion in middle and medial site of lesion.
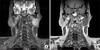
Fig. 3
A. Pre-auricular incision was done. B. Superficial parotid gland was removed and the mass was excised. C. Facial nerve trunk was conserved.

Fig. 4
The tumor cells rarely showed keratinization and almost consisted of intermediate cell type. There were small and cuboidal, exhibiting deeply basophilic nuclei and little cytoplasm and mitotic activity was rarely seen (H&E staining, ×100).
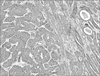
Fig. 5
PCNA was frequently positive in tumor cells, but the immunostain of p53 was rarely stained. Cytokeratin-7 was frequently positive in glandular structure, but the cytokeratin-14 was rarely stained. Snail was frequently positive in tumor cells, but the beta-catenine was rarely stained. Pan-K and S-100 was positively stained, but wnt-1 was negative (×100). (PCNA: proliferating cell nuclear antigen, Pan-K: pancytokeratin)

References
1. Gonzalez-Garcia R, Nam-Cha SH, Munoz-Guerra MF, Gamallo-Amat C. Basal cell adenoma of the parotid gland. Case report and review of the literature. Med Oral Patol Oral Cir Bucal. 2006. 11:E206–E209.
2. Junquera L, Gallego L, de Vicente JC, Fresno MF. Bilateral parotid basal cell adenoma: an unusual case report and review of the literature. J Oral Maxillofac Surg. 2010. 68:179–182.

3. Kawata R, Yoshimura K, Lee K, Araki M, Takenaka H, Tsuji M. Basal cell adenoma of the parotid gland: a clinicopathological study of nine cases--basal cell adenoma versus pleomorphic adenoma and Warthin's tumor. Eur Arch Otorhinolaryngol. 2010. 267:779–783.

4. Esteves AR, Dib LL, de Carvalho LV. Basal cell adenoma: a case report. J Oral Maxillofac Surg. 1997. 55:1323–1325.

5. Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Curr Opin Otolaryngol Head Neck Surg. 2004. 12:127–132.

7. Jones AS, Hamilton JW, Rowley H, Husband D, Helliwell TR. Adenoid cystic carcinoma of the head and neck. Clin Otolaryngol Allied Sci. 1997. 22:434–443.

8. Neville BW. Oral and maxillofacial pathology. 1995. 1st ed. Philadelphia: Saunders.
9. Ellis GL, Auclair PL, Gnepp DR. Surgical pathology of the salivary glands. 1991. Philadelphia: Saunders.
10. Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 2002. 2nd ed. Philadelphia: WB Saunders.
11. Son CW, Kim KW, Kim CH. Study on expression of glycosaminoglycan in adenoid cystic carcinoma. J Korean Assoc Oral Maxillofac Surg. 2004. 30:271–281.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download