Abstract
Objectives
This study sought to evaluate the efficacy of collagen graft materials, as compared to other graft materials, for use in healing calvarial defects in rabbits.
Materials and Methods
Ten mm diameter calvarial defects were made in ten rabbits. The rabbits were then divided into 4 groups: control, autogenous bone graft, SureOss graft, and Teruplug graft. Bone regeneration was evaluated using histological and radiographic methods.
Results
Based on visual examination, no distinct healing profile was observed. At 4 weeks after treatment, histological analysis showed there was no bone regeneration in the control group; however, at 8 weeks after treatment, new bone formation was observed around the margin of the defective sites. In the autogenous bone graft group, new bone formation was observed at 4 weeks after treatment and mature bone was detected around the grafted bone after 8 weeks. In the SureOss graft group, at 4 weeks after treatment, acute inflammatory and multinuclear cells were noted around the grafted materials; at 8 weeks after treatment, a decrease in graft materials coupled with new bone formation were observed at the defective sites. In the Teruplug graft group, new bone formation was detected surrounding the bone margin and without signs of inflammation. There were statistically significant differences observed between the graft and control group in terms of bone density as evidenced by radiographic analysis using computed tomography (P<0.05), particularly for the autogenous bone graft group (P<0.001).
Recently, there have been lots of efforts to restore bone defects in the oral and maxillofacial areas caused by palatoschisis, cyst, tumor, and extraction of impacted teeth or damaged teeth by external injuries. For such regeneration, numerous bone graft materials and bone substitutes have been utilized, and various success rates from those trials have been reported1-5. As the only one with bone formation ability among the bone graft materials in clinical use, autogenous bone has been considered a standard of bone graft materials; it forms new bone through three processes: bone formation, bone induction, and bone conduction2. However, the auto-genous bone graft method may cause some side effects such as patient discomfort, complication, infection, etc., on the donor site3-6.
The advantage of allogenous bone graft materials obtainable from cadaver or other individual living donor of the same species is that they may be utilized immediately whenever necessary; surgery time for taking the bone to be grafted can also be reduced. However, bone tissue taken from another body has the disadvantage of triggering immune responses by acting as antigen on the recipient body2,3,7. Demineralized freeze-dried bone allograft (DFDBA) is considered a typical bone graft substitute together with the freeze-dried bone of allogenous bone graft materials. According to Urist8, new bone formation was induced when bone graft that was freeze-dried after demineralizing cortical bone with hydrochloric acid was applied. Libin et al.9 also reported new bone formation on the grafted bone and osteoblasts near the new bone in their research, wherein they applied DFDB to the human periodontal pocket. Moreover, bone induction is known to increase when collagens and morphogenetic proteins existing within the bone materials are exposed through demineralization3,10,11. Nonetheless, the long-term space-maintaining effect may be reduced since absorption happens too early owing to the resulting lower bone density from the demineralization process12.
There have been many research studies on allogenous bone graft substitutes whose collagen materials have been clinically utilized extensively. The absorbable Atelo-collagen sponge is an expanding material to supplement the bone with collagen, which is inserted into the extraction socket for conservative bone healing using the extraction wound following teeth extraction. It is collagen extracted from cow skin, processed with absorbable heat-treated Atelo-collagen to enhance biocompatibility and to minimize antigenicity. It contains collagen type I (85-95%) and collagen type II (5-15%)2,3,7,13.
To date, there have been lots of studies wherein the observation of bone healing is done after grafting DFDBA to the bone defects2-9. The results of research on the bone formation ability of collagen sponge have also been reported recently, but there has been no study on the comparison of collagen sponge with bone healing after grafting autogenous bone or DFDBA. Accordingly, there is a need to check how effective the bone healing ability of collagen sponge is compared to autogenous bone graft or DFDBA.
Therefore, in this study, bone formation ability was compared and assessed by measuring the computed tomography (CT) numbers, also called Hounsfield Units, showing the relative density based on a straight line scale according to criteria wherein the air dried in the effective scanning energy is set as -1,000, and 25℃ pure water, as 0 after grafting autogenous bone, DFDBA, and collagen sponge to a rabbit cranium defect and performing histological exami nation and dental cone beam computed tomography (CBCT).
Ten New Zealand rabbits that weighed 3 kg or so and which were incubated under identical conditions were utilized as experimental animals.
As experimental materials, autogenous bone, DFDBA (SureOss; Hans Biomed Corp., Seoul, Korea), and collagen sponge (Teruplug; Termo Co., Tokyo, Japan) were used.
Anesthesia was administered by injecting 1 mL/kg each beneath the skin after mixing the 1 : 1 mixture agents of tiletamine and zolazepam (Zoletil; Virbac Corp., Carros, France) and xylazine (Rumpun; Bayer Korea, Seoul, Korea) at a ratio of 1 : 1.
After getting rid of the hairs on the cranium of the rabbit, 1-2 amples of 2% hydrochloric acid lidocaine (Huons Co., Seongnam, Korea) containing 1 : 100,000 epinephrine were injected under the skin for hemostasis. After making the cranium exposed by cutting the scalp with a No.15 medical operation knife, 4 circular defects with diameter of 10 mm were created. Extreme care was taken to avoid damaging the cerebral meninges using the 10-mm diameter trephine bur.(Fig. 1)
The non-treated bone with defect became the control group; the defected bone grafted with autogenous bone taken from the cranium was the autogenous cranium graft group, the defected bone grafted with SureOss (0.15 cc), the SureOss group, and the defected bone grafted with Teruplug (15×4 mm), the Teruplug group. A complete wound closure was performed by sealing with 3-0 Monosyn (B.Braun, Rubi, Spain), an absorbable surgical suture.(Fig. 2)
After 4 weeks and 8 weeks of experiment, 5 rabbits were sacrificed from each group, and four bone defect sites were extracted and fixed in 10% formalin solution after the cranium defect sites were exposed carefully without damaging them.
After 4 and 8 weeks of experiment, the experimental area of the sacrificed experimental animals was visually examined.
After the cranium defects fixed in formalin solution were demineralized with ethylenediaminetetraacetic acid solution and embedded with paraffin, 5 µm tissue slices were made and stained with H&E. The histological properties were then observed using an optical microscope (Nikon Inc., Melville, NY, USA).
After putting all the calvarial defect sites fixed in formalin solution into the container filled with water, the average value was obtained after measuring the CT number 5 times in 10-mm diameter using the V-works program (Cybermed Inc., Seoul, Korea) against the image acquired with CBCT (CB Mercuray; Hitachi, Tokyo, Japan) D-mode (voxel 0.1×0.1×0.1 mm3, 120 kv, 15 mA).(Fig. 3)
After calculating the average and standard deviation against the measured values of CT number as acquired from each specimen, statistical analyses were performed using the Mann-Whitney U test for the comparison of water and control group, Kruskal-Wallis one-way analysis of variance for the comparison of the control group and the experimental group, and Wilcoxon signed rank test for the comparison of the values obtained after 4 and 8 weeks.
As a result of visual examination, uniform healing conditions were not observed. However, there was no big difference; healing proceeded without any exposed bone defect site or separation or major inflammation in the cut.
The bone defect sites were filled with connective tissues, and normal blood vessels and bone formation by osteoblasts were partially observed. No inflammation was found, however. Slight bone formation was observed even in the marginal area of the bone defect site, but most of the middle part of the bone defect area was filled with connective tissues; neovascularization was also noted.(Fig. 4)
The autogenous cranium grafted on the bone defect site was found to be intact without findings of bone absorption or inflammation, with neovascularization noted around the grafted bone.(Fig. 5)
The grafted SureOss was well-held together, and it secured effective room. Acute inflammation cells such as neutrophils and numerous multinucleated giant cells encircled the graft materials on their surroundings. The proliferation of new blood cells was noted around the graft materials. New bone was generated from the marginal area of the bone defect site, and osteoblasts were observed around the graft materials near the new bone.(Fig. 6)
Neither special inflammation on the bone defects nor graft material was found. New bone was observed on the marginal area of the bone defects, with the central area filled with connective tissues.(Fig. 7)
The bone defect sites did not show a big difference from those in the 4th week, but new bone formation was noted on the marginal site of the bone defects. No inflammation was observed, and most of the bone defects were filled with connective tissues.(Fig. 8)
Matured new bones were found more around the graft bone compared to the 4th week; osteoblasts surrounded the new bone densely. Bone formation on the defective site was taking place without any inflammation.(Fig. 9)
SureOss decreased compared to the 4th week, and no inflammation was found. Proliferation of new bone around the grafted bone was noted, including new bone formation around the grafted bone and the marginal area of the bone defects as well.(Fig. 10)
Slight inflammation was noted on the bone defects, with no material used as graft material found. Neovascularization was observed on the existing bone at the marginal site of the bone with defect.(Fig. 11)
In the CBCT image after 4 weeks, radiolucent bone defects were observed from the control group. From the autogenous cranium graft group, radiopaque bone formation was found on the most marginal area, and comparatively dense bone (high-density bone) was noted. From the SureOss graft group, radiopaque bone formation was observed on some part of the marginal area, including bone formation with uneven density in the inside. From the Teruplug graft group, low-density bone formation limited to its marginal area was noted.(Fig. 12)
In the CBCT image after 8 weeks, weak radiopaque bone formation was found in the marginal area for the control group; the internal status of bone defects was also seen. In some parts of the autogenous cranium graft group, bones whose density was similar to that of the surround/ing normal bones were noted. In some parts of the SureOss graft group, bones whose density was similar to that of the surrounding normal bones were found, and absorption of the grafted bone was partially observed. For the Teruplug graft group, internal radio-opacity increased compared to the 4th week.(Fig. 13)
The measured CT number values of the control group and the experimental groups showed that the control group had average value of -176.14, minimum of -285.76, and maximum of -90.22 after 4 weeks; the autogenous cranium graft group had average value of 326.70, minimum of 259.52, and maximum of 377.50, whereas the SureOss graft group had average value of -45.76, minimum of -125.44, and maximum of 45.78. Finally, the Teruplug graft group had average value of -17.60, minimum of -129.54, and maximum of 135.58.
The control group had average value of -163.31, minimum of 257.52, and maximum of -24.80 after 8 weeks; the autogenous cranium graft group had average value of 333.72, minimum of 215.58, and maximum of 435.76. On the other hand, the SureOss graft group had average value of 42.74, minimum of -154.74, and maximum of 206.38. Finally, the Teruplug graft group had average value of 53.62, minimum of -54.32, and maximum of 209.58.
In the comparison of CT number values after 4 weeks and 8 weeks between water and control group, no statistically significant difference was noted (P>0.05). In contrast, there were statistically significant differences after 4 weeks and 8 weeks between the 3 experimental groups and the control group (P<0.05). For the autogenous graft group in particular, statistically significant differences were remarkably shown after 4 weeks and 8 weeks compared with other experimental groups (P<0.001). For the CT number after 4 weeks and 8 weeks, there was no statistically significant difference between the SureOss graft group and the Teruplug graft group (P>0.05).
No statistically significant difference was found after 4 weeks and 8 weeks between the control group and each of the 3 experimental groups (P>0.05).(Table 1)
In the oral and maxillofacial areas, various bone defects are caused by teeth extraction, palatoschisis, cyst, tumor, etc. Consequently, functional and esthetic problems follow, and installation of dental implants, and orthodontic treatment are performed to solve such problems. Thus, appropriate bone shape is very important above all. In particular, as dental implant installation has become popular, and interest in esthetic prosthetics has been surging, the healing aspects of the extraction socket following teeth extraction have attracted considerable interest, and clinical efforts have been made to maintain the height and width of the alveolar bone before taking out teeth. However, naturally healed extraction socket may easily have inappropriate shape for dental implant insertion and aesthetic prosthetics since a reduction of socket height and width may occur simultaneously during bone formation within the extraction socket of the alveolar bone14-16. Thus, bone graft using various kinds of graft materials has been performed to restore bone defect sites. Experimental and clinical research studies seeking the best treatment method of restoring bone defects in the oral and maxillofacial area have been attempted, and efforts to find an ideal treatment method have been made continuously1-7.
The various bone graft materials being applied on bone defect sites form bones through three independent processes: bone formation, bone induction, and bone conduction2,3,17,18. Bone-forming graft materials directly form and grow bones, come from tissues connected with the growth and healing of bones or include some parts of the tissues, and stimulate the growth of bones as well as in soft tissues. The graft materials for bone induction increase bone regeneration after grafting and make the bone grow even into the area where there is no bone. Bone conduction graft materials work as a physical substrate in new bone formation and facilitate the deposition and growth of bone in the substrate bone but lack the ability of bone formation in soft tissues19.
Autogenous bone has been considered the gold standard as the only type with bone forming ability. Hislop et al.6 evaluated highly the autogenous bone graft material's bone forming ability among many bone graft materials, citing its good conditions as a dental bone graft material. The bone absorption rate in case of grafting membranous bone such as cranium, mandibular menton and ramus, etc. of autogenous bone that can be taken for grafting is known to be lower than the absorption rate in case of grafting the endochondral bone such as rib, ilium, etc.20. In the histological findings of this study, bone absorption was not observed on the bone defects of the autogenous cranial bone, and new bone and neovascularization were noted around the grafted bone. After 8 weeks in particular, matured new bones were formed around the grafted autogenous bone, showing appropriate and good regeneration on the bone defects. The average value in the radiological findings of this study was 326.70 in the 4th week and 333.72 in the 8th week, showing statistically significant differences compared with those of other experimental groups (P<0.001). Autogenous cranium bone was deemed to show the best bone formation ability.
As bone induction material, DFDBA may improve bone induction capability by exposing collagens and morphogenetic proteins existing within the bone materials through demineralization3,10,11. In the study of graft wherein DFDBA was applied to rat calvarial bone, the graft materials' movement was reported to be less, thereby maintaining the space21. Senn22 attempted to increase bone forming ability by removing the minerals hindering chemical bone induction through demineralization. Kalish et al.23 reported a meaningful bone regeneration effect in the study of graft using DFDBA wherein polyol was applied as substrate to the defects of a rat. In the histological findings of the graft group to which SureOss as the DFDBA used in this study was grafted, meaningful bone formation on the bone defects was observed. The graft materials were effective in maintaining the space after 4 weeks, but acute inflammation cells such as neutrophils and numerous multinucleate cells were found around some parts of the graft materials. In the 8th week, neovascularization was observed around the grafted bone together with the reduction of the graft materials grafted on the bone defects. Neovascularization was noted around the grafted bone and at the marginal site on the bone defects as well. The observation of multinucleated giant cells and inflammation in this study are believed to be attributable to the responses against foreign materials. In the radiological findings of this study, the average value of CT numbers was -45.76 in the 4th week and 42.74 in the 8th week, which were meaningfully lower compared with those of the control group (P<0.05).
Collagen in nature has insoluble tropocollagen structure as a protein existing in skin, tendon, and bone, and it may be used as hemostatic in nature. However, telopeptide, its component, may work as antigenic material between different kinds. Accordingly, to increase biocompatibility, telopeptide is removed from tropocollagen and subsequently processed into soluble Atelo-collagen and neutralized into the fibrillar Atelo-collagen having structure similar to that of collagen in nature. Finally, heat-denatured Atelo-collagen with high biocompatibility is made through heat treatment at 37℃ or higher. The Teruplug used in this study is made by mixing the heat-denatured Atelo-collagen and the fibrillar Atelo-collagen at the ratio of 1 : 912,24. In the study of collagen materials, Koide et al.25 said that the structure of fibrillar Atelo-collagen was more similar to that of collagen compared with Atelo-collagen, and that the fibrillar type has strong mechanical resistance. The heat-denatured Atelo-collagen has lower resistance compared to Atelo-collagen but works well for the attachment and activity of cell. In the research on the construction of collagen materials, Konishi26 reported that better results may be produced for the mechanical strength and attachment and activity of cells when fibrillar collagen and heat-denatured Atelo-collagen are used in mixture than when Atelo-collagen, fibrillar collagen, and heat-denatured Atelo-collagen are used individually. However, there may be some differences in effectiveness depending on the ratio of mixture. In the histological findings of this study, the Teruplug graft group showed neovas cularization on the marginal site of bone defects in the 4th week, but the group showed slight inflammation and neovascularization on the existing bone in the 8th week. Such result is thought to be in accordance with the report of Kim et al.13, i.e., when Teruplug is applied, bone generation may be accelerated compared with the control group, inducing the proliferation of neighboring cells and increasing new bone formation from the surrounding bones through bone conduction. In the radiological findings of this study, the average value of CT numbers was -17.6 in the 4th week and 53.62 in the 8th week, showing statistically significant differences compared with those of other experimental groups. The values in the 4th week and 8th week showed no meaningful differences between the SureOss graft group and the Teruplug graft group, however.
Based on the experimental results, autogenous bone, DFDBA, and collagen sponge were all effective in bone formation. In particular, the bone formation ability of collagen sponge was limited compared with autogenous bone but was similar to that of DFDBA, and complete generation of bone was still not observed even in the 8th week.
Since the experiments in this study were performed in the given environment of rabbit cranium unlike the oral environment that varies depending on numerous factors, the bone healing aspects to be shown when graft materials are directly applied to the oral defects experienced by other animals or humans must be compared and observed in future studies.
This study was conducted to determine through the comparison how effective the healing ability of collagen sponge is compared with autogenous bone and other bone grafting materials. After artificially making 10 mm diameter bone defects on the cranium of a rabbit weighing 3 kg or so, it was compared and analyzed that the bone forming ability of 5 rabbits for each group from the control group for which no treatment was done and other experimental groups for which autogenous cranium, DFDBA (SureOss), and collagen sponge (Teruplug) were grafted.
In the visual examination, no healing aspect was observed. There was neither large difference among four different groups nor meaningful finding. In the histological findings, bone formation was not observed at the marginal area of the bone defects in the 4th week in the control group, but neovascularization was noted at the marginal area in the 8th week. In the autogenous bone graft group, new bone was formed around the grafted bone, and matured new bone was observed in the 8th week compared to the 4th week. In the SureOss graft group, acute inflammation cells and multinucleated giant cells were found around the grafted bone in the 4th week, and reduction of graft materials and neovascularization were observed around the graft materials and at the marginal site of the bone defects in the 8th week. In the Teruplug graft group, neovascularization was observed at the marginal site of bone without any inflammation finding. The CT number values depending on the X-ray absorption amount in proportion to the density of tissues in the radiological examination. Based on the statistical analysis, the values were significantly higher in all experimental groups, particularly for the autogenous graft group (P<0.001), than in the control group (P<0.05).
In the aforesaid findings, bone formation ability was shown to be higher in the autogenous bone graft group, the SureOss graft group, and the Teruplug graft group than in the control group. The bone forming ability of Teruplug was lower than autogenous bone but similar to that of SureOss.
Figures and Tables
Fig. 2
The bony defected area were grafted with autogenous bone (A), SureOss (0.15 cc) (B), and Teruplug (15×4 mm) (C) separatedly in the rabbit.
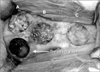
Fig. 3
Example of computed tomography (CT) number of the defected sites measured using V-works program. (W: CT number of the water, G: CT number of the cranial defects)

Fig. 4
Photomicrographs of the control group in the 4th week showing loose connective tissue filled the space and little bone formation in the marginal area of the bone defect site (H&E staining, A: ×10, B: ×50).

Fig. 5
Photomicrographs of the autogenous cranium grafted group in the 4th week showing newly formed bone (N) around the grafted bone (H&E staining, A: ×10, B: ×50).

Fig. 6
Photomicrographs of the SureOss (S) graft group in the 4th week showing effective space maintenance of the graft material and emerging acute inflammatory cells and multinuclear cells (MC, arrow) around the graft material and newly formed bone from the marginal area of the bone defect site (H&E staining, A: ×10, B: ×50).

Fig. 7
Photomicrographs of the Teruplug graft group in the 4th week showing the loose connective tissues filled the bone defects and newly formed bone (N) (H&E staining, A: ×10, B: ×50).

Fig. 8
Photomicrographs of the control group in the 8th week showing loose connective tissues filled the defected site and new bone (N) formation from the edge of the bone defect (H&E staining, A: ×10, B: ×50).

Fig. 9
Photomicrographs of the autogenous cranial bone graft group in the 8th week showing mature bone (MB) and new bone (N) around the grafted autogenous bone (H&E staining, A: ×10, B: ×50).

Fig. 10
Photomicrographs of the SureOss (S) graft group in the 8th week showing decreased volume of the graft materials and newly formed bone (N) from surrounding of the graft materials and marginal area of the bone defects as well (H&E staining, A: ×10, B: ×50).

Fig. 11
Photomicrographs of the Teruplug graft group in the 8th week showing newly formed bone (N) at the marginal site of the bone with defect (H&E staining, A: ×10, B: ×50).

Fig. 12
Axial cone beam computed tomography images of control group (A) and study groups (B-D) in the 4th week. The control group shows bone defect (A), the autogenous cranial bone graft group shows radiopacity of high-density bone (B), the SureOss graft group shows the radiopacity at part of the periphery and internal area (C), and the Teruplug graft group shows radiopacity limited to its marginal area (D).

Fig. 13
Axial cone beam computed tomography images of control group (A) and study groups (B-D) in the 8th week after bone graft. The control group shows bone formation at the periphery (A), the autogenous cranial bone graft group shows increased radiopacity (B), the SureOss graft group shows radiopacity and radiolucency (C), and the Teruplug graft group shows increased radiopacity in the internal area (D).

Table 1
Computed tomography number of calvarial defects
(WA: water, CG: control group, AB: autogenous calvarial bone graft group, SO: SureOss graft group, TP: Teruplug graft group)
*Significant (P<0.05), **Highly significant (P<0.01), ***Extremely significant (P<0.001).
The Mann-Whitney U test was used for comparing between water and control group.
Kruskal-Wallis one-way analysis of variance was used for comparing between the control group and the three graft groups.
The Wilcoxon signed rank test was used for comparing between the results 4 and 8 weeks after the graft.
References
1. Lee EJ, Chung HJ. Histologic study on healing after implantation of several bone substitutes in rat calvarial defects. J Korean Acad Periodontol. 1998. 28:87–102.

3. Lane JM. Bone graft substitutes. West J Med. 1995. 163:565–566.
4. Rummelhart JM, Mellonig JT, Gray JL, Towle HJ. A comparison of freeze-dried bone allograft and demineralized freeze-dried bone allograft in human periodontal osseous defects. J Periodontol. 1989. 60:655–663.

5. Kim SH, Kim CK, Chai JK, Cho KS. Clinical study on therapeutic effects of decalcified freeze dried bone allograft in intrabony defects. J Korean Acad Periodontol. 1994. 24:618–632.
6. Hislop WS, Finlay PM, Moos KF. A preliminary study into the uses of anorganic bone in oral and maxillofacial surgery. Br J Oral Maxillofac Surg. 1993. 31:149–153.

7. Schepers EJ, Ducheyne P, Barbier L, Schepers S. Bioactive glass particles of narrow size range: a new material for the repair of bone defects. Implant Dent. 1993. 2:151–156.
8. Urist MR. Bone: formation by augmentation. Science. 1965. 150:893–899.
9. Libin BM, Ward HL, Fishman L. Decalcified, lyophilized bone allografts for use in human periodontal defects. J Periodontol. 1975. 46:51–56.

10. Acil Y, Springer IN, Broek V, Terheyden H, Jepsen S. Effects of bone morphogenetic protein-7 stimulation on osteoblasts cultured on different biomaterials. J Cell Biochem. 2002. 86:90–98.

11. Wikesjo UM, Sorensen RG, Kinoshita A, Wozney JM. RhBMP-2/alphaBSM induces significant vertical alveolar ridge augmentation and dental implant osseointegration. Clin Implant Dent Relat Res. 2002. 4:174–182.
12. Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. Histologic evaluation of new attachment apparatus formation in humans. Part III. J Periodontol. 1989. 60:683–693.
13. Kim JH, Kim CH, Kim KW. Bone healing capacity of the collagen bone filler (TERUPLUG(R)) and rhBMP-2 in the rabbit cranium defect. J Korean Assoc Oral Maxillofac Surg. 2008. 34:119–130.
14. Lekovic V, Kenney EB, Weinlaender M, Han T, Klokkevold P, Nedic M, et al. A bone regenerative approach to alveolar ridge maintenance following tooth extraction. Report of 10 cases. J Periodontol. 1997. 68:563–570.

15. Lang N, Becker W, Karring T. Lindhe J, editor. Alveolar bone formation. Textbook of clinical periodontology and implant dentistry. 1998. 3rd ed. Copenhagen: Munksgaard;906–932.
16. Irinakis T, Tabesh M. Preserving the socket dimensions with bone grafting in single sites; an esthetic surgical approach when planning delayed implant placement. J Oral Implantol. 2007. 33:156–163.

17. Frame JW. Hydroxyapatite as a biomaterial for alveolar ridge augmentation. Int J Oral Maxillofac Surg. 1987. 16:642–655.

18. Pinholt EM, Bang G, Haanaes HR. Alveolar ridge augmentation in rats by combined hydroxylapatite and osteoinductive material. Scand J Dent Res. 1991. 99:64–74.

19. Sepe WW, Bowers GM, Lawrence JJ, Friedlaender GE, Koch RW. Clinical evaluation of freeze-dried bone allografts in periodontal osseous defects--part II. J Periodontol. 1978. 49:9–14.

20. Koole R, Bosker H, van der Dussen FN. Late secondary autogenous bone grafting in cleft patients comparing mandibular (ectomesenchymal) and iliac crest (mesenchymal) grafts. J Craniomaxillofac Surg. 1989. 17:Suppl 1. 28–30.

21. Matzenbacher SA, Mailhot JM, McPherson JC 3rd, Cuenin MF, Hokett SD, Sharawy M, et al. In vivo effectiveness of a glycerol-compounded demineralized freeze-dried bone xenograft in the rat calvarium. J Periodontol. 2003. 74:1641–1646.

22. Senn On the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Ann Surg. 1889. 10:352–368.
23. Kalish BP, Schuster GS, Peacock ME, Cuenin MF, Swiec GD, Potter BJ, et al. Influence of matrix-suspended demineralized bone on osseous repair using a critical-sized defect in the rat (Rattus norvegicus) calvarium. J Oral Implantol. 2008. 34:83–89.

24. Makoto H, Nobuyuki M, Shinjiro A, Susumu O, Kei W, Tomomichi O, et al. Efficacy of tooth extraction wound protection made of atelocollagen sponge (TRE-641): a pilot study in dogs. J Hard Tissue Biol. 2009. 18:89–94.

25. Koide M, Osaki K, Konishi J, Oyamada K, Katakura T, Takahashi A, et al. A new type of biomaterial for artificial skin: dehydrothermally cross-linked composites of fibrillar and denatured collagens. J Biomed Mater Res. 1993. 27:79–87.

26. Konishi J. Composition of collagen materials. Jpn J Artif Organs. 1989. 18:155–158.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download