Abstract
Herpes zoster is a viral infection caused by the reactivation of the varicella zoster virus, an infection most commonly affecting the thoracolumbar trunk. Herpes Zoster Infection (HZI) may affect the cranial nerves, most frequently the trigeminal. HZI of the trigeminal nerve distribution network manifests as multiple, painful vesicular eruptions of the skin and mucosa which are innervated by the infected nerves. Oral vesicles usually appear after the skin manifestations. The vesicles rupture and coalesce, leaving mucosal erosions without subsequent scarring in most cases. The worst complication of HZI is post-herpetic neuralgia; other complications include facial scarring, motor nerve palsy and optic neuropathy. Osteonecrosis with spontaneous exfoliation of the teeth is an uncommon complication associated with HZI of the trigeminal nerve. We report several cases of osteomyelitis appearing on the mandible, caused by HZI, and triggering osteonecrosis or spontaneous tooth exfoliation.
The varicella zoster virus (VZV) produces two clinical results: varicella or chickenpox and herpes zoster infection (HZI)1. Varicella caused by the primary infection of VZV is a benign childhood disease producing eruptive vesicles. As a result of primary infection in the varicella, a skin virus moves to a sensory nerve and remains in latent state in a ganglion1,2. When VZV in latent state is reactivated, it develops into HZI, which causes severe pain and painful vesicles in the skin and mucosa around the affected sensory nerve distribution3,4.
Thoracolumbar dermatomes (T3-L3) are most commonly affected by HZI1,4. HZI may also affect the cranial nerves, with the trigeminal nerve most frequently affected (18.5-22%)1. Herpes zoster affecting the trigeminal nerve is generally unilateral; it affects a single branch among three branches, mainly the first branch or optic nerve. Oral manifestation can be observed when the maxillary and mandibular branches are affected4.
Oral vesicles appear mainly after a skin manifestation3,5. Sometimes, however, there may be mucosal involvement without skin lesion5. The vesicles erupt and leave mucosal erosions but no scar in most cases3. The most significant complication of HZI is post-herpetic neuralgia5; other complications may include facial scarring, motor nerve palsy, optic neuropathy, blindness, encephalitis, and calcinosis cutis6.
The bony change in association with HZI was first reported by Rose in 19087. According to Dechaume et al. (1955)8, Gonnet's presentation in 1922 was the first report to establish interest in osteonecrosis and tooth exfoliation associated with HZI2. Complication such as osteonecrosis with spontaneous tooth exfoliation is very rare. Thus, we report some cases of osteomyelitis in the mandible caused by HZI affecting the trigeminal nerve.
A 78-year-old male patient visited our hospital on February 12, 2010 with chief complaint of osteonecrosis of mandible after the extraction of the mandibular right canine in a private dental clinic. The mandibular right first premolar and second premolar were said to have exfoliated naturally during a meal and with toothbrushing two months before he came to the hospital, and there were no symptoms such as pain or mobility in the past. Four weeks before he came to the hospital, the skin lesion appeared along the pathways of the maxillary/mandibular branches in the right trigeminal nerve, and antiviral agent was given in a private skin clinic for symptom reduction, however, paresthesia in right chin appeared. After a manifestation of skin lesion, the patient complained of pain and mobility of the mandibular right canine and the canine was extracted in a private dental clinic. Following diagnosis of impaired healing and osteonecrosis, the patient was referred to our department.
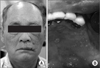
A skin lesion in the mandibular right area was observed, showing a healing pattern based on the clinical examination.(Fig. 1) The oral findings included exposure of necrotic bone in the mandibular right area, pus discharge through extraction sockets for canine, first and second premolar, and malodor.(Fig. 2) The patient's oral hygiene was poor and there were many root rests observed in the maxillary right second molar, first molar, second premolar, first premolar, maxillary left first molar, mandibular left first premolar, second premolar, and first molar. There was no significant finding from the blood test and chemical test. Additionally, computed tomography (CT) scan was conducted. The radiological findings included a low attenuate intraosseous lesion with illdefined border. Reactive osteosclerosis around the lesion, and sequestrum were seen in a lesion. Other findings included the bony destruction pattern of the buccolingual cortical bone near the lesion and multiple enlarged lymph node in the submandibular space.(Fig. 3)
When the patient visited the hospital again on the February 17, the exposure pattern of cortical bone in the right mandible showed no significant change compared to the first clinical examination, and the CT findings were explained to the patient and his caregiver. We temporarily diagnosed it as mandibular osteomyelitis caused by HZI, hospitalized the patient on the 18th of the same month and year, and planned antibiotherapy, consultation with dermatologist, and sequestrectomy.
The dermatologist decided not to require additional antiviral treatment because the skin lesion had already entered the healing stage, and just recommend the administration of non-steroidal anti-inflammatory drug (NSAID) and pregabalin to provide relief from post-herpetic neuralgia. With antibiotherapy, squestrectomy and extraction of root rests were performed under local anesthesia on February, 26. The bony defects were dressed with Vaseline gauze, which was replaced on a daily basis. The postoperative radiological findings showed normal healing pattern of the area where the bone was removed.(Fig. 4) The lesion was overlapped with soft tissue in good condition nine days after the operation, and the patient dischargedthe hospital on the 16th day after the operation. As a result of monitoring until eight months after the surgery, the patient did not complain of post-herpatic neuralgia, and there were no findings of recurrence.
A 77-year-old male patient was referred to our hospital with chief complaint of sore gingiva and mandibular pain on December 17, 2010. A month before visiting our hospital, the patient complained of pain in the right ear area, vesicles and swellings with pain along the pathway of the trigeminal mandibular branch on the right side of the face, and consulted an otolaryngologist, taking antiviral agent medication. Two weeks before he visited our hospital, he felt pain from sore gingiva and exposure of cortical bone beneath the gum. He had used maxillary complete denture and mandibular removable partial denture for several years.
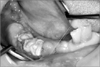
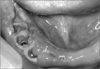
The clinical examination revealed findings of erythematous edema in the right chin and ulcer and post-inflammatory hyperpigmentation in the right chin and preauricular area.(Fig. 5) The oral findings included the extraction socket (considered to have been dropped recently) of the mandibular right canine, first and second premolar used as abutment of the removable partial dentures, exposure of neighboring necrotic bones, and inflammation of neighboring gingiva.(Fig. 6) The patient did not remember the exact time of tooth exfoliation, but it was estimated to be about one week earlier according to the opinions of his caregiver and the people around him. Assuming mandibular osteomyelitis in association with HZI, antibiotics were prescribed to prevent secondary infection, and CT scan was conducted. Consultation with the dermatologist and otolaryngologist was administered for the skin lesion and otalgia. The radiological finding showed a low attenuate intraosseous lesion with relatively ill-defined borderin the mandibular right area but no destruction of cortical bone or sequestrum. Some of the enlarged lymph node in the submandibular space was observed.(Fig. 7) Dermatologically, since one month had passed after HZI, antiviral treatment was not required. For post-herpatic neuralgia, pregabalin and acetaminophen were administered.
When he visited the hospital again, the exposure pattern of the right mandibular cortical bone had no significant change compared to the first clinical examination. Since there was neither sequestrum nor acute inflammation, we only use antibiotics and antimicrobials (Chlorhexidine Gluconate Solution) for prevent secondary infection. Surgical squestrectomy was planned in case of sequestrum develops in the future. Continuous follow-up and disinfection were performed; since post-herpatic neuralgia persisted, gabapentin 600 mg, tramadol 37.5 mg, acetaminophen 325 mg, and aminotriptyline 10 mg were administered to the patient.
On June 8, 2011, after continuous follow-up for six months, post-herpatic neuralgia subsided, and sequestrum formed and naturally exfoliated. Biopsy of the exfoliated sequestrum was performed, with the result reported as necrotic bone. On June 29, the area was overlapped with soft tissue around the lesion, healing well.(Fig. 8)
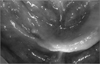
On July 22, 2010, a 74-year-old male patient visited our hospital with chief complaint of swelling of the left side of the face and pain. He complained of pain in the upper anterior teeth for three days before he visited our hospital, and vesicles and edema were formed along the pathway of the left trigeminal maxillary branch two days before he visited our hospital. On July 22, he went to a private dental clinic; he was then referred to our hospital. He took prescription pills for high blood pressure, which was relatively well-regulated. He wore maxillary and mandibular partial dentures for five years. The clinical examination showed that the left side of the face had diffuse erythematous plaques and vesicles with tenderness.(Fig. 9. A); the oral findings included multiple mucosal vesicles and ulcer formation on the left palate together with mobility of the remaining teeth and alveolar bone resorption in edentulous state except the maxillary right and left central incisor, lateral incisor, and canine.(Fig. 9. B) HZI was considered to be in active phase, consultation were administered with a dermatologist and a periodontist to prevent osteonecrosis and loss of remaining teeth. After the treatment by a dermatologist, antiviral agent (famciclovir 750 mg), tramadol 37.5 mg, acetaminophen 325 mg, levocetirizine HCl 5 mg, and mupirocin 0.2% ointment were prescribed; for periodontal treatment, scaling and monitoring were performed. On July 29, the skin lesion showed normal healing pattern by the formation of crust (Fig. 10. A); the oral vesicles also healed normally with no significant scar.(Fig. 10. B) On August 26 of the same year, monitoring revealed no major abnormality except moderate mobility of the remaining maxillary teeth, and that post-herpatic neuralgia subsided.
The prevalence rate of HZI in all ages is reported to be 1.2-4.8 per 1,000 people every year, with 7.2-11.8 people over the age of 609. The prevalence rate and seriousness increase with age. 40-50% of the patients with HZI are over the age of 60 every year, with 50% of persons over 85 years old recording prevalence rate of at least once10. As to the reason for such prevalence rate increase, natural immunity decline according to age increase may be considered; the decline in VZV-specific cellular immunity according to age increase is supported1,10. The prevalence rate also increases among immunocompromised patients such as patients infected with human immunodeficiency virus, hematologic malignant disease, and immune-mediated disorder and organ transplant patients, and the risk of HZI for such immune-suppressed patients also increases according to age1,9,10. Other risk elements may include external damage of the affected dermatomes, psychological stress, and race9.
The thoracic dermatome is most commonly affected, accounting for 50% of the total cases9. The cranial nerve may also be affected, with the trigeminal nerve most commonly affected (18.5-22% of the total cases) followed by glossopharyngeal nerve and hypoglossal nerve. In case of trigeminal nerve involvement, it is unilaterally limited to a single branch, mainly affecting the optic nerve2.
Oral symptom is observed when the trigeminal maxillary and mandibular branches are affected and skin lesion is mainly preceded, but a case starting with paresthesia of mental nerve was also reported11. The erythematous vesicle is developed in the oral cavity; it ruptures, forms an ulcer, and gets covered by white pseudomembrane2,5,11. Lymphadenopathy may appear in the submandibular area5. In addition, patients may complain of symptom similar to acute pulpits of the affected tooth and toothache12; root resorption and periapical lesion may also occur13. Since histological findings that are not significantly different from the osteomyelitis pattern show necrotic bone and inflam matory cell infiltration, the diagnosis of the relationship with herpes zoster can hardly be confirmed only by such histological findings; the diagnosis should take into account the clinical findings3,4,14. Among the findings, osteonecrosis of the jaw and natural tooth exfoliation are very rare complications4,5,15. Jain et al.4 conducted a review of literature on 41 cases of osteonecrosis of the jaw caused by HZI. The onset age range was from 6 to 85, with 8 cases of patients under the age of 40, 10 cases of those between 40 and 60, and 12 cases of those over 60. The prevalence rate increased according to age, with no difference by gender4,16. It mainly appeared unilaterally in the maxilla or mandible of the affected skin. 13 patients had it in the maxilla, and 18 patients, in the mandible. There were 31 lost teeth in the maxilla and 44 teeth in the mandible, making the mandible a predilection site. Since the anterior teeth numbered 64 and the posterior teeth were 61, there was no significant difference in the anteroposterior position. The exfoliated teeth per patient were 0-7; in five cases, all the teeth of the affected quadrant were lost4.
With regard to the time interval between the outbreak of HZI and osteonecrosis accompanied by tooth loss, Mintz and Anavi3 presented in 1992 a report on the interval of an average of 21.2 days together with the occurrence of natural tooth exfoliation about 2-6 weeks after HZI manifestation through a review of 14 studies. Several authors5,11 reported that it occurred between two weeks after early infection with complication of osteonecrosis and tooth exfoliation, but other authors4 reported that it occurred between 3-12 weeks after HZI manifestation as a late complication. In the first case reported in this paper, the extraction of the mandibular right canine was performed about 4 weeks after HZI manifestation even though the mandibular first and second premolars were ruled out because those premolars were lost four weeks before the outbreak of skin lesion. In the second case, natural tooth exfoliation occurred about three weeks after HZI manifestation.
Taking into account the comparison of general osteomyelitis, in case of findings that show a medical history of herpes zoster recently affecting the trigeminal nerve and vesicular lesion in the related facial area with proper interval, and that the lesion is limited only to quadrant of jaw with the affected nerve distributed, it may be diagnosed as osteomyelitis of the jaw caused by trigeminal herpes zoster. Patients showing osteomyelitis of the jaw in Case 1 and Case 2 were old, taking prescription pills for high blood pressure and having medical history of coronary artery bypass surgery to avoid angina pectoris and cerebral artery infarction, respectively. Since they were old and weak patients with blood diseases, it might be diagnosed as general chronic pyogenic osteomy elitis caused by local dental infection. In Case 1, however, many remaining teeth were affected by dental caries or root rests; since the radiopaque lesions around the root apex were distributed in the maxilla and mandible, it could be judged that there was local dental infection causing chronic osteomyelitis. Taking into account the findings of
The pathophysiological mechanism of osteomyelitis of the jaw caused by trigeminal herpes zoster is controversial, and several hypotheses are under discussion. The first hypothesis is that local vasculitis caused by the direct extension of neural inflammatory response to the adjacent blood vessels. This eventually may cause an infarction of the trigeminal vessels that accompany the trigemical nerves supplying the jaws and cause bone necrosis by triggering ischemia16. The second hypothesis is that the generalized infection of terminal nerves supplying the periosteum and periodontium is believed to cause vasculitis of the periosteum and periodontium and avascular necrosis over a large area5,15,17. According to Gilden et al.18, since VZV can also be invaginated to the vas cular endothelial cell in the peripheral nervous system instead of being limited to the brain or spinal cord, it causes small ischemic lesion, possibly developing into necrosis and demy elination. The report can serve as grounds for the two hypotheses above18. The third hypothesis can be a denervation of bone, but denervation only can hardly be thought to cause serious bone damage in a short time15. The fourth hypo thesis is that systemic viral infection renders damage to an odontoblast and brings about tissue denaturation, causing pulpal necrosis. The final hypothesis is that the existing pulpits, periodontitis, or surgical procedure around the HZI area can cause serious necrosis of the alveolar bone3. In comparing Case 1, Case 2, and Case 3 above, the regulation of initial response and topical factors is meaningful in preventing complications such as osteomyelitis and osteonecrosis of the jaw, and the local factor can be considered a cause of the mechanism in the development of osteomyelitis associated with trigeminal herpes zoster.
The cases above and literature review reveal that, in case of the outbreak of trigeminal herpes zoster, the prompt application of antiviral agents, active use of painkillers, and effective regulation of topical factors will be helpful in preventing such complications1,4,9,10. If osteomyelitis and osteonecrosis of the jaw occur as complications, such complications can be treated through the proper use of antibiotics to avoid secondary infection, squestrectomy, removal of inflammatory tissue, and regular follow up2,4,13-15. In these cases, when osteomyelitis of the jaw and osteonecrosis accompanied by tooth exfoliation as rare complications after the outbreak of trigeminal herpes zoster occur, active use of painkillers, regulation of topical factors, and proper extraction of dead bone and affected teeth bring about good results.
Figures and Tables
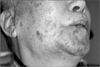 | Fig. 1Case 1. Skin lesions of herpes zoster (healing phase); clusters on the skin on the lower side of the face (V3 area) were observed. |
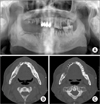 | Fig. 3Case 1. Preoperative radiographic findings. A. Large sequestrum was seen on the right mandible, and it was definitely distinguished from the surrounding bone on the orthopantograph. B. Mandibular computed tomography (CT) axial view; large sequestrum can be seen on the right mandible. C. Mandibular CT axial view; exfoliated socket can be seen on #33, 34, 35. |
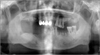 | Fig. 4Case 1. Post-operative radiographic findings post-operative day 8; the necrotic bone and multiple hopeless teeth were removed, and normal healing was noted. |
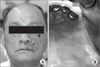
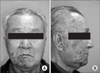 | Fig. 5Case 2. Skin lesions of herpes zoster; erythematous swelling and ulcer with post-inflammatory hyperpigmentation. A. Skin lesion can be seen on the chin, right. B. Skin lesion can be seen on the preauricular area, right. |
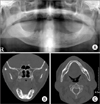 | Fig. 7Case 2. Radiographic findings; diffuse radiolucent lesion and extraction sockets area seen on the mandible, right. A. Orthopantograph. B. Mandibular computed tomography (CT) view: coronal. C. Mandibular CT view: axial. |
References
1. Gershon AA, Gershon MD, Breuer J, Levin MJ, Oaklander AL, Griffiths PD. Advances in the understanding of the pathogenesis and epidemiology of herpes zoster. J Clin Virol. 2010. 48:Suppl 1. S2–S7.

2. Mendieta C, Miranda J, Brunet LI, Gargallo J, Berini L. Alveolar bone necrosis and tooth exfoliation following herpes zoster infection: a review of the literature and case report. J Periodontol. 2005. 76:148–153.

3. Mintz SM, Anavi Y. Maxillary osteomyelitis and spontaneous tooth exfoliation after herpes zoster. Oral Surg Oral Med Oral Pathol. 1992. 73:664–666.

4. Jain MK, Manjunath KS, Jagadish SN. Unusual oral complications of herpes zoster infection: report of a case and review of literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010. 110:e37–e41.

5. Muto T, Tsuchiya H, Sato K, Kanazawa M. Tooth exfoliation and necrosis of the mandible--a rare complication following trigeminal herpes zoster: report of a case. J Oral Maxillofac Surg. 1990. 48:1000–1003.

6. Owotade FJ, Ugboko VI, Kolude B. Herpes zoster infection of the maxilla: case report. J Oral Maxillofac Surg. 1999. 57:1249–1251.

7. Rose F. Postherpetic neuralgia; trophic lesions in hand's bones similar to rheumatoid arthritis. Nouvelle Iconogr de la Salpêtrière. Soc Neurol. 1908. 9:64. [French].
8. Dechaume M, Descrozailles C, Garlopeau F, Robert J. Localized mandibular necrosis during trigeminal herpes. Revue Stomatol. 1955. 56:516–521. [French].
9. Schmader KE, Dworkin RH. Natural history and treatment of herpes zoster. J Pain. 2008. 9:1 Suppl 1. S3–S9.

10. Weaver BA. Herpes zoster overview: natural history and incidence. J Am Osteopath Assoc. 2009. 109:6 Suppl 2. S2–S6.
11. Schwartz O, Kvorning SA. Tooth exfoliation, osteonecrosis of the jaw and neuralgia following herpes zoster of the trigeminal nerve. Int J Oral Surg. 1982. 11:364–371.

12. Sigurdsson A, Jacoway JR. Herpes zoster infection presenting as an acute pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995. 80:92–95.

13. Ramchandani PL, Mellor TK. Herpes zoster associated with tooth resorption and periapical lesions. Br J Oral Maxillofac Surg. 2007. 45:71–73.

14. Arikawa J, Mizushima J, Higaki Y, Hoshino J, Kawashima M. Mandibular alveolar bone necrosis after trigeminal herpes zoster. Int J Dermatol. 2004. 43:136–137.

15. Garty BZ, Dinari G, Sarnat H, Cohen S, Nitzan M. Tooth exfoliation and osteonecrosis of the maxilla after trigeminal herpes zoster. J Pediatr. 1985. 106:71–73.

16. Wright WE, Davis ML, Geffen DB, Martin SE, Nelson MJ, Straus SE. Alveolar bone necrosis and tooth loss. a rare complication associated with herpes zoster infection of the fifth cranial nerve. Oral Surg Oral Med Oral Pathol. 1983. 56:39–46.

17. Hall HD, Jacobs JS, O'Malley JP. Necrosis of maxilla in patient with herpes zoster. Report of a case. Oral Surg Oral Med Oral Pathol. 1974. 37:657–662.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download