Abstract
Objectives
This study evaluated the clinical results of partial sublingual glandectomy accompanying the excision of ranula as new treatment modality.
Materials and Methods
A total of 43 patients who were treated between 1999 and 2007 for oral or plunging ranula were reviewed. All patients were treated surgically by various methods with a total of 55 different procedures performed. Ten cases of partial sublingual glandectomy with excision of the ranula were conducted. All excised specimens were examined. We compared the clinical outcomes resulting from each treatment method.
Results
The recurrence rates for marsupialization, excision of ranula, marsupialization with gauze packing, total excision of sublingual gland and ranula, and partial sublingual glandectomy with excision of ranula were 50%, 25%, 25%, 0% and 10%, respectively. Of the 10 patients treated by partial sublingual glandectomy with ranula excision, only one experienced recurrence (10%), i.e., plunging ranula. None of the ranulas contained an epithelial lining, and the excised portion of the feeding sublingual glands showed degenerative changes.
Conclusion
In removal of ranulas, we found that excision of the attached sublingual gland, which removed the feeding portion and degenerative acinar cells, yielded good outcomes. Thus, as a new conservative method for treatment, we recommend partial sublingual glandectomy to accompany excision of the ranula.
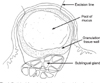
A ranula is a mucocele occurring on the floor of the mouth and is derived either from the extravasation of saliva out of the salivary ducts or retention of saliva inside the salivary ducts, originating in the minor salivary gland or the sublingual gland as one of the major salivary glands1,2. In general, a ranula is known as an extravasation phenomenon of the sublingual gland3-6. It looks like a cystic lesion, but it is a pseudocyst without epithelial lining3,7-12. Note, however, that there have been few cases wherein mucus retention cysts of Rivini and Wharton ducts have been reported1. In this case, a true epithelial lining made of the epithelium of the duct was detected. When a ranula spreads out from the posterior inferior area of the mylohyoid muscle to the submental area or cervical area, it is known as a plunging ranula. In general, ranulas appear as a bluish dome-shaped swelling on the floor of the mouth. When mucoceles accumulate in the subcutaneous area or near the epidermis area, a ranula appears as a light bluish color because of epidermal cyanosis and retained saliva in the subcutaneous area. When a ranula exists in the deeper areas, however, the mucosa appears as an ordinary pinkish color.
A ranula does not cause serious symptoms of pain except some discomfort, and it hardly gives rise to any severe clinical manifestation. According to Baurmash1, clinical findings such as discomfort in speech, mastication, and swallowing and external swelling differ depending on the size and location of the ranula. In the case of a very large mucocele in the sublingual gland, the tongue may compress the ranula during eating and swallowing such that there is interference with the salivary flow of the submandibular gland. When a plunging ranula increases in size, it may cause dyspnea and dysphagia and may expand as far as the mediastinum13. There are several surgical methods for the treatment of ranulas, with varying cure rate7,8,14. Although a simple technique, marsupialization has been reported to have a high recurrence rate. To minimize any recurrence, Baurmash2 proposed marsupialization with gauze packing and suggested that the removal of the entire sublingual gland could be overtreatment in some cases. In contrast, there have been several reports of the extirpation of the entire sublingual gland, recording a relatively high cure rate. Thus, the removal of the entire sublingual gland is proposed to minimize any recurrence7,8,11,14-16. Note, however, that the complete removal of the sublingual gland may involve the functional deterioration of the salivary glands and increase the possibility of damage to the Wharton's duct, lingual nerves, and blood vessels6,16.
In this research, we have investigated the usefulness of a procedure involving the simultaneous partial removal of a damaged area of the sublingual gland with the excision of the ranula. This method maintains the function of the sublingual gland and minimizes the recurrence rate. Here, we describe partial sublingual glandectomy with the excision of the ranula as a new treatment model and compare it with other surgical procedures.
Retrospective evaluation was performed on 43 consecutive patients diagnosed with ranulas during the period 1999-2007 at the authors' institution. The patients' ages ranged from 3 to 75 years, with average age of 21 years. A total of 16152 surgical treatments were performed on the 43 patients.(Table 1) Mucoceles in areas other than the floor of the mouth and occurrences on the Wharton's duct were excluded. Based on the medical records, we investigated the diagnosis, treatment, status of relapse, and complications. The results of each surgical procedure were reviewed, and we assessed the clinical results of partial sublingual glandectomy with the excision of the ranula in comparison with the other methods used. Histological examinations were performed on all excised specimens. Each ranula was completely excised, except in the case of marsupialization. The follow-up period was from at least six months up to two years.
Among the 43 patients, 10 were treated with partial sublingual glandectomy with excision of the ranula. Surgery was performed under general anesthesia. Elliptical incision was made on the mucosa of the ranula. Meticulous, blunt dissection was performed along with the pseudocyst up to the sublingual gland. When dissection reached the body of the sublingual gland secreting the ranula, the location of the attachment portion of the sublingual gland was confirmed and excised for 1-1.5 cm.(Fig. 1) The resected margin of the sublingual gland was sutured with 4-0 non-absorbable silk, and the incised mucosa was closed using a general method. The other procedures used-except partial glandectomy-were carried out using ordinary methods.
At least 39 and 4 patients were diagnosed with ranulas on the floor of the mouth and plunging ranulas, respectively. There were 24 cases of excision of the ranula, 10 cases of marsupialization, 4 cases of marsupialization with gauze packing, 4 cases of total excision of the sublingual gland and ranula, and 10 cases of partial sublingual glandectomy with excision of the ranula. The recurrence rates were 25%, 50%, 25%, 0%, and 10%, respectively.
Eight patients underwent partial sublingual glandectomy with excision of the ranula as the first operation, and one of the patients suffered from recurrence.(Table 2) This male patient was 3 years old and was diagnosed with a ranula at the first visit. One month after the surgery, the patient had a recurrent lesion. The patient was rediagnosed with a plunging ranula and subsequently treated with total excision of the sublingual gland and ranula.
In two of the patients who were each treated with excision of the ranula and marsupialization in the first surgery, ranulas recurred one month later. These patients were operated on using partial sublingual glandectomy (Fig. 2), and neither suffered from recurrence. The recurrent lesions were treated with partial sublingual glandectomy with excision of the ranula, and the condition was resolved.
In one case of excision of the ranula, the patient complained of parethesia on the ipsilateral side of the tongue immediately after the surgery, but the symptoms were resolved two weeks later with no further treatment. Other than post-operative swelling or discomfort, no other complications were found.
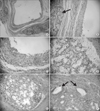
According to the histological tests, no lesions with true epithelial lining were found. The mucus cavity was lined with loose connective tissue and granulation tissue. These showed fibrous tissue, inflammatory cells, foamy histiocytes, and lymphocytes in the connective tissue. Degeneration of acinic cells from partially excised sublingual glands and dilation of the duct were observed.(Fig. 3) Inflammation and degeneration were detected in the sublingual gland adjacent to the ranula.
Ranula is a clinical term denoting a mucocele occurring on the floor of the mouth. The etiology of a ranula is discussed in terms of ductal obstruction and damage of the salivary duct17. The retention of mucus and ductal dilation occur with the obstruction of this duct. The result is an epithelial-lined cyst. Observation of these cases is rare; the size of the lesion is also small8. The common form of a ranula is a pseudocyst derived from the extravasation of the sublingual gland3-17. The reason for the extravasation is considered to be trauma to the sublingual gland and related structure1,7,8. When the acinic cells are damaged by trauma, saliva leaks into adjacent tissue and forms a cystic cavity lined by granulation tissue surrounded by loose connective tissue. Many studies have confirmed that the excised ranula has no true epithelial lining3,7-11. The etiology for a ranula is still not clear, however1,18. In this research, no lesions with true epithelial lining were observed; thus, ranulas are thought to be an extravasation phenomenon of the sublingual gland.
There have been several methods used for the treatment of ranulas, but proper modality is still a subject of debate because of the tendency toward recurrence. The recurrence rates in literature are summarized in Table 3, decreasing in the following order: marsupialization, excision of ranula, and excision of the sublingual gland. Zhao et al.14 stated that the recurrence rates of ranulas were not related to the patterns of lesion but were closely related to the method of the surgical procedure used. Crysdale et al.8 suggested excision of the sublingual gland for ranulas >1 cm. Catone et al.11 and Bridger et al.15 proposed excision of the sublingual gland as the primary treatment for all ranulas regardless of size.
Because of its simplicity and complications after the excision of the sublingual gland, many clinicians prefer marsupialization as the first treatment16,19. Complications associated with the surgical management of ranulas have been investigated by Zhao et al.16. According to them, the most frequent complications are the recurrence of lesion and numbness of the tongue due to damage to the lingual nerve. Damage to the Wharton duct, hemorrhaging, hematoma, wound dehiscence, and infection have been reported as well. Recurrence was frequent in the marsupialization and excision of the ranula. Injury to adjacent tissue was related mainly to the excision of the sublingual gland. Note, however, that these complications after the excision of the ranula were temporary and were resolved within a given timeframe. Thus, to minimize recurrence as the most common complication, the removal of the sublingual gland has been suggested.
Some clinicians are still skeptical as to the total removal of the sublingual gland. Baurmash5 insists that the total excision of the sublingual gland is not appropriate for small-sized ranulas and suggests a modified marsupialization with gauze packing. Baurmash used this method on 12 cases, with 1 case of recurrence reported. According to Baurmash, however, marsupialization cannot resolve ranulas associated with the deep part of the sublingual gland. In addition, marsupialization (either once or repeated) can cause fibrosis of the upper side; thus, mucus can be induced in the lower part of the lesion, resulting in a plunging ranula in case of recurrence15.
To summarize the viewpoint of the healing process, the previously reviewed treatment method can be categorized as follows: chronic fistulization of the lesion; sealing of the feeder base; excision of the ranula only, and; removal of the sublingual salivary gland as the origin. Marsupialization forms a chronic fistula between the mucus secretion part and the oral cavity. Note, however, that most ranulas do not have a true epithelial lining; thus, a chronic fistula cannot be established8,11. Consequently, there is recurrence with the healing of the oral epithelium. The technique of sealing the feeder base induces atrophy and fibrosis of the damaged part of the sublingual gland. Marsupialization with gauze packing, cauterization using CO2 laser20,21, and injection of picibanil (OK-432)22,23 fall under this category. Marsupialization accompanied by gauze packing maintains a channel to the oral cavity and allows time for fibrosis of the mucus secretion area; hence its lower recurrence rate than simple marsupialization. Similarly, fibrosis and sclerosis of the mucus secretion part are induced by CO2 laser and injection of OK-432. In this trial, however, the possibility of recurrence still exists because the damaged part of the sublingual gland is not excised. The procedure for the excision of the ranula only removes the cyst, so it has a blind point, i.e., it can miss the feeder gland. The removal of the sublingual gland as the source of the pseudocyst is a radical treatment; hence its potential complications.
Partial sublingual glandectomy with excision of the ranula can satisfy many of the requirements discussed above. First, it removes the ranula lesion as well as the origin of the lesion by excising the mucus-supplying part of the sublingual gland. Suturing of the excised margin of the sublingual gland is intended to seal the feeder base. Furthermore, the sublingual gland has several secretion ducts that open independently; thus, functional recovery of the sublingual gland can be expected even after the excision of part of the sublingual gland. In addition, it is noninvasive compared to the total extirpation of the sublingual gland, and so it is expected to have fewer complications.
Partial glandectomy is mainly applied to operations on the parotid gland, and superficial parotidectomy is practiced widely. Partial superficial parotidectomy has been introduced as a more conservative method than superficial parotidectomy24. This partial salivary glandectomy is based on the regenerative ability of the salivary glands, and the healing procedure of damaged salivary glands of rodents has been reported25,26. Note, however, that there have been no reports on partial sublingual glandectomy. Similar to the regeneration of parotid glands, sublingual glands are expected to go through a similar healing procedure.
In this study, we have shown that patients who underwent partial sublingual glandectomy with the excision of a ranula recovered without any noticeable complication and with low recurrence rate.
Therefore, partial sublingual glandectomy with excision of the ranula has value in clinical practice as a new conservative method. Nonetheless, the number of cases in this research is still small, so further investigations are needed. Moreover, further research on factors such as the amount of excision, functional recovery, and change in the pattern of sublingual glands after partial excision is required.
Figures and Tables
Fig. 2
Gross findings of excised ranula and excised portion of sublingual gland. Solid arrow: ranula, hollow arrow: attached sublingual gland.

Fig. 3
Histopathologic features of ranula. A, B. A cystic cavity is surrounded by loose connective tissue or granulation tissue (H&E staining, A: ×50, B: ×120). Adjacent feeding sublingual gland (arrow) is seen near the cavity. C. The cystic wall is composed of numerous inflammatory cells, foamy histiocytes, lymphocytes, and fibrous tissue (H&E staining, ×150). D, E. In the sublingual gland, acinic cells show degenerative change (H&E staining, ×400). F. Ducts (arrows) are dilated and degenerated (H&E staining, ×500).
References
2. Baurmash HD. A case against sublingual gland removal as primary treatment of ranulas. J Oral Maxillofac Surg. 2007. 65:117–121.

3. Kurabayashi T, Ida M, Yasumoto M, Ohbayashi N, Yoshino N, Tetsumura A, et al. MRI of ranulas. Neuroradiology. 2000. 42:917–922.

4. Shelley MJ, Yeung KH, Bowley NB, Sneddon KJ. A rare case of an extensive plunging ranula: discussion of imaging, diagnosis, and management. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002. 93:743–746.

5. Baurmash HD. Marsupialization for treatment of oral ranula: a second look at the procedure. J Oral Maxillofac Surg. 1992. 50:1274–1279.

6. Baurmash HD. Treating oral ranula: another case against blanket removal of the sublingual gland. Br J Oral Maxillofac Surg. 2001. 39:217–220.

7. Yoshimura Y, Obara S, Kondoh T, Naitoh S. A comparison of three methods used for treatment of ranula. J Oral Maxillofac Surg. 1995. 53:280–282.

8. Crysdale WS, Mendelsohn JD, Conley S. Ranulas--mucoceles of the oral cavity: experience in 26 children. Laryngoscope. 1988. 98:296–298.
9. Galloway RH, Gross PD, Thompson SH, Patterson AL. Pathogenesis and treatment of ranula: report of three cases. J Oral Maxillofac Surg. 1989. 47:299–302.

10. Morita Y, Sato K, Kawana M, Takahasi S, Ikarashi F. Treatment of ranula--excision of the sublingual gland versus marsupialization. Auris Nasus Larynx. 2003. 30:311–314.

11. Catone GA, Merrill RG, Henny FA. Sublingual gland mucus-escape phenomenon--treatment by excision of sublingual gland. J Oral Surg. 1969. 27:774–786.
12. Barnes L. Surgical pathology of the head and neck. 1985. 1st ed. New York: Marcel Dekker;1297–1301.
13. Marx RE, Stern D. Oral and maxillofacial pathology. 2003. Illinois: Quintessence Publishing;511.
14. Zhao YF, Jia Y, Chen XM, Zhang WF. Clinical review of 580 ranulas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004. 98:281–287.

15. Bridger AG, Carter P, Bridger GP. Plunging ranula: literature review and report of three cases. Aust N Z J Surg. 1989. 59:945–948.

16. Zhao YF, Jia J, Jia Y. Complications associated with surgical management of ranulas. J Oral Maxillofac Surg. 2005. 63:51–54.

17. Quick CA, Lowell SH. Ranula and the sublingual salivary glands. Arch Otolaryngol. 1977. 103:397–400.

18. Harrison JD, Garrett JR. An ultrastructural and histochemical study of a naturally occurring salivary mucocele in a cat. J Comp Pathol. 1975. 85:411–416.

19. Chidzonga MM, Mahomva L. Ranula: experience with 83 cases in Zimbabwe. J Oral Maxillofac Surg. 2007. 65:79–82.

20. Frame JW. Removal of oral soft tissue pathology with the CO2 laser. J Oral Maxillofac Surg. 1985. 43:850–855.

21. Mintz S, Barak S, Horowitz I. Carbon dioxide laser excision and vaporization of nonplunging ranulas: a comparison of two treatment protocols. J Oral Maxillofac Surg. 1994. 52:370–372.

22. Woo JS, Hwang SJ, Lee HM. Recurrent plunging ranula treated with OK-432. Eur Arch Otorhinolaryngol. 2003. 260:226–228.

23. Fukase S, Ohta N, Inamura K, Aoyagi M. Treatment of ranula wth intracystic injection of the streptococcal preparation OK-432. Ann Otol Rhinol Laryngol. 2003. 112:214–220.

24. Martis C. Parotid benign tumors: comments on surgical treatment of 263 cases. Int J Oral Surg. 1983. 12:211–220.

25. Milstein BB. Regeneration in the submaxillary gland of the rat. Br J Exp Pathol. 1950. 31:664–669.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download