Abstract
Background
Management of pregnant women at high risk of pre-eclampsia (PE) requires frequent monitoring, with referral to specialized perinatal care centers. Reliable tests are necessary to improve prediction of PE and related complications and to assess disease severity and progression. An imbalance in two biomarkers, soluble fms-like tyrosine kinase 1 (sFlt-1) and placental growth factor (PlGF), is involved in PE pathogenesis. The sFlt-1 to PlGF ratio is increased in pregnant women before the onset of PE. An elevated ratio is highly predictive of PE, whereas the diagnosis of PE can be ruled out within one week for low ratios. The main objective of this study was to assess whether a low sFlt-1/PlGF ratio, below a cutoff of 38, can predict the absence of PE within one week.
Methods
We performed a prospective, monocentric, observational study to evaluate serum sFlt-1/PlGF ratio (Roche Diagnostics Cobas e411 system) for predicting -PE in a group of 67 high-risk pregnant women (20–37 gestation weeks).
Results
Among the 67 patients included, 53 had a sFlt-1/PlGF ratio lower than 38; none developed subsequent PE leading to a negative predictive value of 100%. Eight patients developed clinical PE. The positive predictive value was 21% at one week and 18% at four weeks, in accordance with previous studies.
Pre-eclampsia (PE) is a heterogeneous disease defined by the onset of hypertension and proteinuria after 20 weeks' gestation, affecting 2–5% of pregnancies worldwide [123]. The clinical presentation and outcome of PE is variable, and early-onset severe and rapidly progressive PE with pre-term delivery to late-onset PE have been described [4]. PE remains an important cause of maternal and fetal/neonatal morbidity and mortality. Indeed, severe and life-threatening complications such as intrauterine growth restriction (IUGR), eclampsia, and HELLP syndrome (hemolysis, elevated liver enzymes, and low platelet count) can occur [4]. Clinical management can be challenging because of the lack of effective treatment options other than delivery.
The pathogenesis of PE is multifactorial and not fully understood. However, abnormal remodeling of maternal spiral arteries is observed in women with subsequent PE, leading to placental hypoperfusion and systemic endothelial dysfunction [5]. Placental dysfunction leads to altered circulating levels of pro-angiogenic or anti-angiogenic mediators, notably placental growth factor (PlGF), soluble endoglin and the soluble fms-like tyrosine kinase receptor-1 (sFlt-1), and a truncated and soluble form of vascular endothelial growth factor (VEGF) receptor, which neutralizes VEGF and PlGF. Hence, PE and its complications may occur because of an imbalance in circulating angiogenic factors [6].
Levine et al [7] showed that five weeks before development of clinical symptoms of PE, serum concentrations of s-Flt-1 are increased, whereas PlGF concentrations are decreased, resulting in an increased s-Flt-1/PlGF ratio. Increased sFlt-1 levels and reduced PlGF levels can be used to predict the subsequent development of PE.
While increasing data are becoming available and promising results have been observed for sFlt-1 and PlGF, soluble endoglin and VEGF have not been shown to be clinically effective for managing PE. Despite its pathophysiological implications, no specific increase in soluble endoglin in the context of PE or HELLP syndrome has been demonstrated [8]. The use of VEGF as a tool for managing PE appears to also be compromised. There are analytical difficulties in the measurement of VEGF because of the different assays available for total or free, biologically active VEGF measurement [9].
The potential relevance of determining s-Flt-1, PlGF, and the s-Flt-1/PlGF ratio for the diagnosis and prognosis of the disease has been investigated to help physicians identify patients at high risk of PE who require close monitoring. Interestingly, the sFlt-1/PlGF ratio appears to show better performance than single markers in predicting the risk of PE [1011]. Several strategies have been investigated for first trimester screening of women at high risk of PE to introduce, if applicable, low-dose acetylsalicylic acid and during the second trimester or later, to predict or rule out the diagnosis of PE [12].
Additionally, a major prospective clinical trial validated cut-offs for the sFlt-1/PlGF ratio for the management of PE in the PROGNOSIS study (Prediction of Short-Term Outcome in Pregnant Women with Suspected Preeclampsia Study), which clearly demonstrated that an sFlt-1/PlGF ratio of 38 or lower can be used to rule out the onset of PE within one week, independently of gestational age [13]. The negative predictive value was 99.3% [13]. Furthermore, a sFlt-1/PlGF ratio greater than 85 (for early-onset PE, <34 weeks of gestation) or 110 (for late-onset PE, ≥34 weeks) is indicative of a high risk of PE diagnosis or placenta-related disorders requiring close clinical and biological monitoring. For women with a sFlt-1/PlGF ratio between 38 and 85 or between 38 and 110, current PE can be ruled out; however, these women carry a high risk of developing PE within four weeks [14].
Despite the simplicity of defining PE, which has recently been debated [15], several clinical situations are also relatively complex to assess in ruling out PE diagnosis: women with pre-existing nephropathy and presenting with chronic proteinuria, women with chronic pre-pregnancy hypertension and IUGR, development of hypertension without proteinuria or the opposite, and hypertension with thrombocytopenia or hepatic cytolysis without proteinuria. Because of current difficulties in diagnosing PE and conducting early identification of women at high risk of PE, other biological markers are necessary; sFlt-1 and PlGF appear to be excellent candidates in this context.
The objective of this study was to evaluate the routine use of sFlt-1, PlGF, and the sFlt-1/PlGF ratio in a specific population of high-risk patients, including multiple pregnancies, in clinical practice to improve care and prognosis for these patients.
This mono-centric, prospective, non-interventional study was conducted from January to May 2014 in the specialized perinatal care center of the Department of Gynecology and Obstetrics in Nantes University Hospital, France. Patients did not object to taking part in the study. Written informed consent was obtained from each patient. The local ethics committee of the University Hospital of Nantes approved the study (No. RC13_0283).
All adult patients with ongoing pregnancy between 20 and 37 gestation weeks and with at least one risk factor were eligible (Table 1). Women with a confirmed diagnosis of PE at the specified gestation period were excluded from the study. During the enrollment period, 75 patients were included, and eight were excluded: seven did not meet the inclusion criteria (two for completing over 37 weeks of gestation, and five for already being diagnosed with PE) and one was excluded for medical termination of pregnancy. A total of 67 women were included in the study over 5 months.
Once consent was obtained, an additional blood sample was collected during routine check-up to determine serum sFlt-1 and PlGF concentrations and calculate the sFlt-1/PlGF ratio. Blood was collected in a standard serum tube: samples were centrifuged and stored at −20℃ until analysis. Serum samples were analyzed within six months of storage. sFlt-1 and PlGF concentrations were measured in the Clinical Biochemistry Laboratory of Nantes University Hospital, using an electrochemiluminescence Elecsys immunoassay on a Roche Diagnostics Cobas e411 system (Roche Diagnostics, Mannheim, Germany). Each run was validated by measuring two levels of quality control material prior to starting the experiment. The reference range (5th–95th percentile) was defined by Roche Diagnostics in a cohort of 877 women ongoing normal single pregnancy from nine European centers (Germany, Spain, Austria, Czech Republic, and Switzerland) leading to 1,685 samples analyzed. PlGF and sFlt-1 assays previously demonstrated strong characteristics in terms of inter- and intra-assay precision with total imprecision CV below 5% [16].
The main endpoint was the occurrence of PE with hypertension (≥140/90 mmHg) and proteinuria (≥0.30 g/24 hours) and/or related complications such as HELLP syndrome or eclampsia. The secondary objective of this study was to evaluate the sensitivity, specificity, and negative and positive predictive values of sFlt-1/PlGF ratio of 38, as determined in the PROGNOSIS study, in the specific population of high-risk pregnancies.
In this high-risk population, a prevalence of 15% of PE was expected. Considering a specificity of 95% for the sFlt-1/PlGF ratio with an accuracy of 5%, 86 inclusions were necessary. Taking into account that 2% of the biomarker results would not be analyzable, our goal was to include 88 patients to ensure the desired accuracy.
Data from patients' clinical histories and serum markers of PE were collected in a database using Microsoft Excel 2010 software (Microsoft Corporation, Redmond, WA, United States). All the data in this study are presented as mean±SD. Sensitivity, specificity, and positive and negative predictive values were determined, and a contingency table was prepared. Statistical analysis according to PE status was performed using MedCalc software for Windows, version 15.0 (MedCalc Software, Ostend, Belgium). Kruskal-Wallis test was conducted to analyze continuous variables and Fisher's exact test for categorical variables. A P value below 0.05 was considered statistically significant.
A total of 67 women were included in the study over five months. All women had at least one risk factor. An abnormal uterine artery Doppler (notch or/and high pulsatility index) was the most common risk factor (N=20; 30%). Seven patients (10.5%) with pre-existing isolated proteinuria and four patients (6%) with pre-existing chronic hypertension were included. The mean age was 32 years (±5 years). Inclusion criteria and cohort distribution according to these criteria are shown in Table 1. Among the cohort of 67 patients, eight developed clinical PE: five before 34 gestation weeks, one between 34 and 37 weeks, and two after 37 weeks. The demographic and clinical characteristics of the cohort, shown in Table 2, did not significantly differ between the two groups, except for arterial blood pressure.
sFlt-1 and PlGF concentrations and sFlt-1/PlGF ratio assessments are shown in Table 2. Twenty-two patients (33%) had a sFlt-1 concentration higher than the 95th percentile, with five (62%) in the pre-eclamptic group. For PlGF, eight patients (12%) had a concentration lower than the 5th percentile, with one in the PE group (12%). The mean sFlt-1/PlGF ratio was 36.5±12 for the entire cohort, which was significantly higher (69±13) for women who developed PE than for women without PE (32±25) (P=0.01). Individual results for women with PE are shown in Table 3.
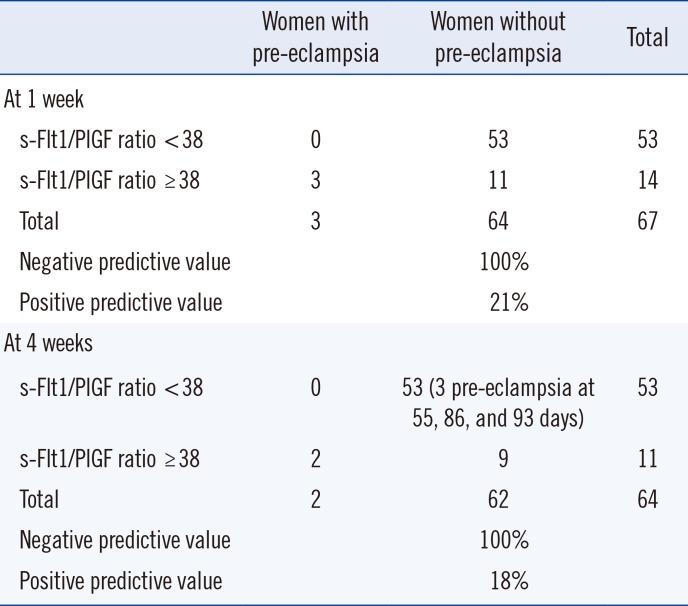
For a sFlt-1/PlGF ratio threshold of 38, 14 women exhibited a high sFlt-1/PlGF ratio (Table 4). Among them, three developed PE within less than one week (two days for one patient, eight days for two patients). The negative predictive value of the sFlt-1/PlGF ratio at one week with a threshold at 38 was 100%; the positive predictive value was 21%. Sensitivity and specificity were 100% and 83%, respectively (Table 4).
Concerning the risk of PE occurring within four weeks at the same threshold and after excluding the three patients who presented with PE before one week, two of the remaining patients developed PE (one patient at 10 days and the other at 31 days). The negative predictive value of the ratio for the risk of PE occurring at four weeks was 100%; positive predictive value was 18%. Sensitivity was 100%, and specificity was 85% (Table 4).
Among the patients who did not develop PE despite a sFlt-1/PlGF ratio greater than 38, three presented with PE but in atypical form: one patient exhibited symptoms of PE with HELLP syndrome but without hypertension, while two patients had PE without proteinuria.
Three patients with a ratio below 38 developed PE at more than four weeks (55, 86, and 93 days).
Our prospective study, conducted in a specialized perinatal care center, demonstrated that the sFlt-1/PlGF ratio can be used to rule out PE in a specific population of high-risk patients with excellent negative predictive value. Our negative predictive value for ruling out PE within one week was 100%, in accordance with that from the PROGNOSIS study (99.3%) [13]. The aim of using the sFlt-1 and PlGF biomarkers is to rule out PE onset, avoiding unnecessary hospitalization and identifying high-risk pregnant women who require intensive monitoring. Use of the two serum biomarkers and their ratio appears to be essential for pregnant women at high-risk of PE or when diagnosis of PE is difficult because of the absence of symptoms or pre-existing diseases.
In fact, hypertensive disorders other than PE such as gestational hypertension or chronic hypertension can occur during pregnancy. The onset of hypertension during pregnancy or worsening of pre-existing chronic hypertension without proteinuria represents a complex situation, in which it is essential to rule out PE. Verlohren et al [17] showed that the sFlt-1/PlGF ratio in patients with PE is significantly higher compared with that in patients with chronic and gestational hypertension. These data, recently confirmed in a Spanish cohort [17], demonstrated the role of the sFlt-1/PlGF ratio in differentiating PE and other pregnancy-related hypertensive disorders [18].
Thus, because both PE and chronic kidney disease (CKD) are characterized by proteinuria, hypertension, and progressive renal impairment, differential diagnosis may be difficult during pregnancy. Rolfo et al [19] evaluated the potential value of using sFlt-1 and PlGF to differentiate between CKD and PE. They demonstrated that women with CKD exhibited normal levels of sFlt-1, PlGF, and sFlt-1/PlGF ratio. The sFlt-1/PlGF ratio was generally significantly higher in subjects with PE than in those with CKD, suggesting that serum biomarkers can be used to distinguish these two diagnoses [19]. Ghosh et al [20] also demonstrated that serum PlGF measured in the early second trimester of pregnancy may be an effective predictor of early-onset PE in another group of high-risk pregnant women who are overweight or obese rather than in normal or underweight women.
Serum sFlt-1 and PlGF PE markers and their ratio show highly predictive performance for clinical care. In addition to the many advantages described above, they are easier to use than uterine artery Doppler and represent an additional method of management in clinical practice. In fact, establishing velocimetric parameters is relatively straightforward. However, uterine artery Doppler has several weaknesses: reproducibility is limited, the resulting indices vary with the anatomical site of insonation, and uterine artery blood flow can be difficult to measure accurately by Doppler sonography [21]. Recent studies have demonstrated the poor sensitivity of using only second-trimester Doppler ultrasound measurements for general prediction of PE in a well-characterized, low-risk, nulliparous population [22]. However, some studies suggested that combining the uterine artery pulsatility index with PlGF and sFlt-1 may be useful for predicting PE [2324].
The actual impact and clinical usefulness of the sFlt-1 and PlGF biomarkers in pregnant women with suspected PE was evaluated in the pre-eclampsia open study (PreOS). sFlt-1/PlGF ratio results led to a change in the decision to hospitalize women for 16.9% of the cohort (20/118). These results demonstrated the usefulness of the sFlt-1/PlGF ratio for clinical decision-making of hospitalization in a considerable proportion of women with suspected PE. In addition to improving clinical care, assessment of the sFlt-1/PlGF ratio helps avoid unnecessary stress and anxiety for the patient. Reducing unnecessary hospitalizations is a major consideration in the current context of a heavy financial burden in many healthcare institutions [25]. The economic impact of introducing sFlt-1 and PlGF for managing PE appears to be positive. In the PROGNOSIS study by Vatish et al [26], hospitalizations were reduced by more than a half, generating cost-savings of £344 per patient. The expected annual cost savings for the UK National Health Service would be approximately £24 million per annum, based on a cohort of 68,900 women presenting annually with hypertensive disorders including suspected PE [26].
Based on this data, the National Institute for Health and Care Excellence (NICE) guidance recommends sFlt-1/PlGF ratio testing to rule out PE in women presenting with suspected PE between 20 and 34+6 gestation weeks. However, there is currently insufficient evidence and some contradictive recent data preventing their routine use in diagnosing PE [2728]. An intermediate and rational method of introducing the sFlt-1/PlGF ratio into clinical practice as a first step, and with respect to the currently relatively high cost, would be to reserve these markers for high-risk populations with suspected PE or for differential diagnosis.
Our study has some limitations, such as the size of the cohort, heterogeneity of the patients included, the mean age of the patients, and influence of multiple pregnancies. Nevertheless, it represents a practical population. The mean age of the patients was 32±5 years with 34 patients out of 67 (50%) aged 30–35 years. Indeed, hypertensive disorders and HELLP syndromes are known to be associated with increasing maternal age during gestation. Concerning multiple pregnancies, 19 patients (28%) in our population had at least a twin pregnancy. Dröge et al [29] reported that women with twin pregnancy who did not develop PE had a higher median serum sFlt-1 concentration and sFlt-1/PlGF ratio than women with singleton pregnancies; however, the median serum PlGF concentration was unchanged. For patients with twin pregnancy and PE, the median sFlt-1/PlGF ratio was not significantly different compared with singleton pregnancies with PE. Current cut-offs for the diagnosis of PE were established using data from singleton pregnancies alone, which, based on our results, appears to be inappropriate: Dröge et al [29] suggested a threshold of 53 as an optimal cut-off for this patient category.
Broader use of the sFlt-1/PlGF ratio in maternity care may support targeted clinical care by helping to identify women who are at high risk of developing PE and require close monitoring and management from women who are at a low risk of developing PE and can simply be reassured, thus avoiding unnecessary hospitalization [25].
This study demonstrated that a cut-off of 38 for the sFlt-1/PlGF ratio is appropriate for ruling out PE in a representative cohort of high-risk patients. Use of the sFlt-1/PlGF ratio in clinical practice will improve management of the disease and reduce health expenditures, while ensuring safety.
Acknowledgements
The authors thank all staff of the Department of Gynecology and Obstetrics. Roche Diagnostics provided assay reagent sets free of charge but did not assume any other role in the conduct of the study. This work was not supported by any research grants.
References
1. Hernandez-Diaz S, Toh S, Cnattingius S. Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ. 2009; 338:b2255. PMID: 19541696.
2. Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002; 346:33–38. PMID: 11778000.
3. World Health Organization. The World Health Report 2005: make every mother and child count. 2014. 11. http://www.who.int/whr/2005/en/.
4. Vogel JP, Souza JP, Mori R, Morisaki N, Lumbiganon P, Laopaiboon M, et al. Maternal complications and perinatal mortality: findings of the World Health Organization Multicountry Survey on Maternal and Newborn Health. BJOG. 2014; 121(S1):76–88. PMID: 24641538.
5. Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009; 24:147–158. PMID: 19509125.
6. Jardim LL, Rios DR, Perucci LO, de Sousa LP, Gomes KB, Dusse LM. Is the imbalance between pro-angiogenic and anti-angiogenic factors associated with preeclampsia? Clin Chim Acta. 2015; 447:34–38. PMID: 25982781.
7. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004; 350:672–683. PMID: 14764923.
8. Hertig A, Liere P. New markers in preeclampsia. Clin Chim Acta. 2011; 411:1591–1595.
9. Jelkmann W. Pitfalls in the measurement of circulating vascular endothelial growth factor. Clin Chem. 2001; 47:617–623. PMID: 11274009.
10. Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, et al. An automated method for the determination of the sFlt-1/PIGF ratio in the assessment of preeclampsia. Am J Obstet Gynecol. 2010; 202:161.e1–161.e11. PMID: 19850276.
11. Rana S, Powe CE, Salahuddin S, Verlohren S, Perschel FH, Levine RJ, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012; 125:911–919. PMID: 22261192.
12. Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet. 2012; 285:417–422. PMID: 21735190.
13. Zeisler H, Llurba E, Chantraine F, Vatish M, Staff AC, Sennstrom M, et al. Predictive Value of the sFlt-1: PlGF Ratio in Women with Suspected Preeclampsia. N Engl J Med. 2016; 374:13–22. PMID: 26735990.
14. Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol. 2015; 45:241–246. PMID: 25736847.
15. Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Pregnancy Hypertens. 2013; 3:44–47. PMID: 26105740.
16. Schiettecatte J, Russcher H, Anckaert E, Mees M, Leeser B, Tirelli AS, et al. Multicenter evaluation of the first automated Elecsys sFlt-1 and PlGF assays in normal pregnancies and preeclampsia. Clin Biochem. 2010; 43:768–770. PMID: 20206155.
17. Perales A, Delgado JL, De La Calle M, Garcia-Hernandez JA, Escudero AI, Campillos JM, et al. sFlt-1/PlGF for early-onset pre-eclampsia prediction: STEPS (Study of Early Pre-eclampsia in Spain). Ultrasound Obstet Gynecol. 2017; 50:373–382. PMID: 27883242.
18. Verlohren S, Herraiz I, Lapaire O, Schlembach D, Moertl M, Zeisler H, et al. The sFlt-1/PlGF ratio in different types of hypertensive pregnancy disorders and its prognostic potential in preeclamptic patients. Am J Obstet Gynecol. 2012; 206:58.e1–58.e8. PMID: 22000672.
19. Rolfo A, Attini R, Tavassoli E, Neve FV, Nigra M, Cicilano M, et al. Is It Possible to Differentiate Chronic Kidney Disease and Preeclampsia by means of New and Old Biomarkers? A Prospective Study. Dis Markers. 2015; 2015:127083. PMID: 26557728.
20. Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S. Serum placental growth factor as a predictor of early onset preeclampsia in overweight/obese pregnant women. J Am Soc Hypertens. 2013; 7:137–148. PMID: 23394804.
21. Kane SC, Dennis AT. Doppler Assessment of Uterine Blood Flow in Pre-eclampsia: a Review. Hypertens Pregnancy. 2015; 34:400–421. PMID: 26389839.
22. Myatt L, Clifton RG, Roberts JM, Spong CY, Hauth JC, Varner MW, et al. The utility of uterine artery Doppler velocimetry in prediction of preeclampsia in a low-risk population. Obstet Gynecol. 2012; 120:815–822. PMID: 22996099.
23. Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S. Combination of uterine artery Doppler velocimetry and maternal serum placental growth factor estimation in predicting occurrence of pre-eclampsia in early second trimester pregnancy: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2012; 161:144–151. PMID: 22280827.
24. Andrietti S, Silva M, Wright A, Wright D, Nicolaides KH. Competing-risks model in screening for pre-eclampsia by maternal factors and biomarkers at 35-37 weeks' gestation. Ultrasound Obstet Gynecol. 2016; 48:72–79. PMID: 26566592.
25. Klein E, Schlembach D, Ramoni A, Langer E, Bahlmann F, Grill S, et al. Influence of the sFlt-1/PlGF Ratio on Clinical Decision-Making in Women with Suspected Preeclampsia. PLoS One. 2016; 11:e0156013. PMID: 27243815.
26. Vatish M, Strunz-McKendry T, Hund M, Allegranza D, Wolf C, Smare C. The sflt-1/plgf ratio test in pre-eclampsia: an economic assessment for the UK. Ultrasound Obstet Gynecol. 2016; 48:765–771. PMID: 27300726.
27. Dragan I, Georgiou T, Prodan N, Akolekar R, Nicolaides KH. Screening for pre-eclampsia using sFlt-1/PlGF ratio cut-off of 38 at 30-37 weeks' gestation. Ultrasound Obstet Gynecol. 2017; 49:73–77. PMID: 27619203.
28. NICE. National Institute for Health and Care Excellence (NICE) Clinical Guidance. PlGF-based testing to help diagnose suspected pre-eclampsia (Triage PlGF test, Elecsys immunoassay sFlt-1/PlGF ratio, DELFIA Xpress PlGF 1-2-3 test, and BRAHMS sFlt-1 Kryptor/BRAHMS PlGF plus Kryptor PE ratio). 2016.
29. Droge L, Herraiz I, Zeisler H, Schlembach D, Stepan H, Kussel L, et al. Maternal serum sFlt-1/PlGF ratio in twin pregnancies with and without pre-eclampsia in comparison with singleton pregnancies. Ultrasound Obstet Gynecol. 2015; 45:286–293. PMID: 25491901.
Table 1
Cohort distribution according to the inclusion criteria
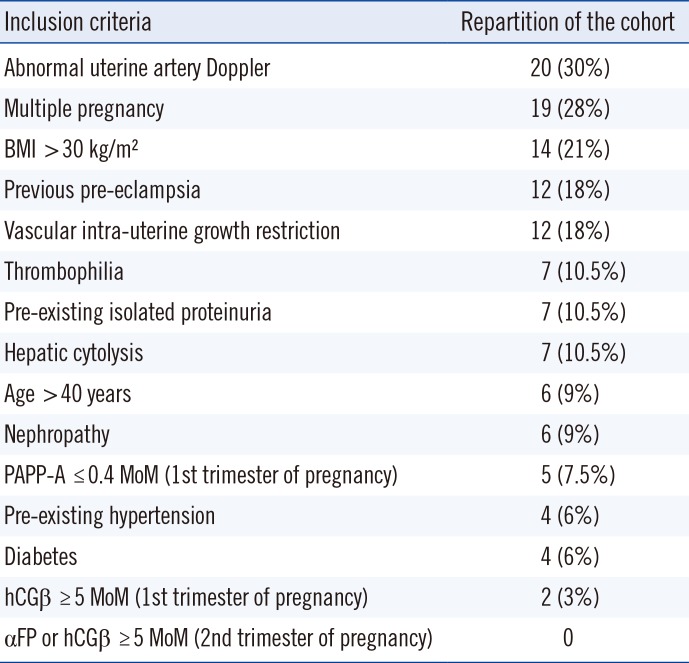
Table 2
Demographical data, clinical characteristics, serum sFlt-1, PlGF concentrations, and sFlt-1/PlGF ratios of patients
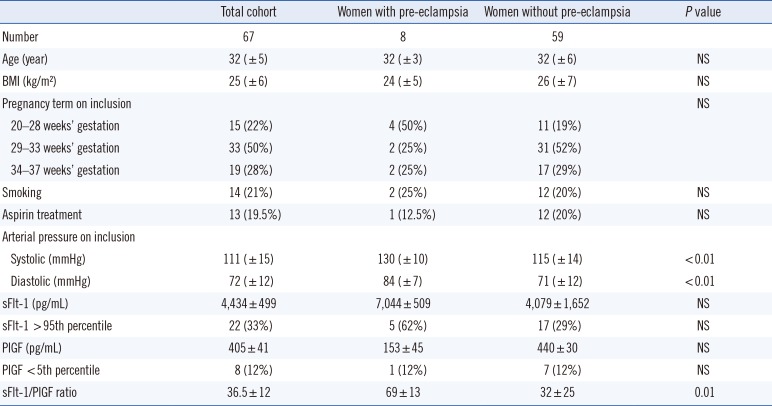
Table 3
Individual results of the eight patients who developed pre-eclampsia
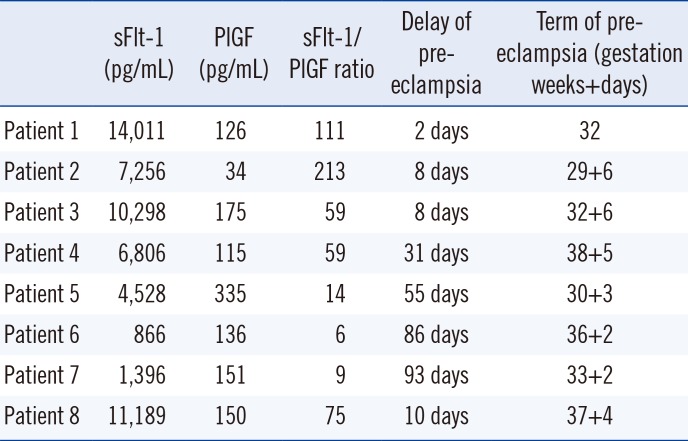
Table 4
Predictive performance of s-Flt1/PlGF ratio




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download