Dear Editor,
Intraabdominal infections are well-known sources of polymicrobial
bacteremia [12]. Anaerobes such as Bacteroides spp. and Clostridium spp. account for 30??0% of these cases [234]. However, a significant proportion of anaerobes remain unidentified. Lee et al [5] reported that the conventional identification method correctly identifies anaerobic bacteria only 79.4% to the genus level and 60.1% to the species level. Here, we report a case of polymicrobial bacteremia with three anaerobes in a patient with peritonitis following intestinal perforation. The anaerobes included Butyricimonas virosa and Brachyspira pilosicoli, both of which are difficult to identify by the conventional identification method [67].
A 68-year-old man was admitted to the hospital with epigastric pain, which gradually exacerbated over three days. His last defecation was two days prior to admission, and he had no diarrhea. The patient had a history of two colorectal surgeries: one for gastric cancer (pylorus side resection) four years ago, and the other for primary carcinoma of the appendix (laparoscopic appendectomy) one year ago. Upon admission, his abdomen was hard with rebound tenderness, and urgent abdominal computed tomography scans revealed abdominal free air and ascites. Thus, we diagnosed him with peritonitis due to gastrointestinal perforation. We performed an emergency operation under general anesthesia after sampling two blood culture specimens. During surgery, we found that the patient's transverse colon was split 10 cm lengthwise and partially punched, and stool was leaking from this opening. The perforation was thought to be due to limited intestinal range of motion caused by a postoperative adhesion. The perforation was sutured, and an artificial anus was constructed via separation of the terminal ileum. The suture ruptured postoperatively, and the patient received intensive care including continuous hemodialysis and vasopressors; he received doripenem for three weeks. His overall condition improved daily, and he was moved from the intensive care unit to the general surgical ward on postoperative day 13. Informed written consent was obtained from the patient for presenting the clinical data in this article.
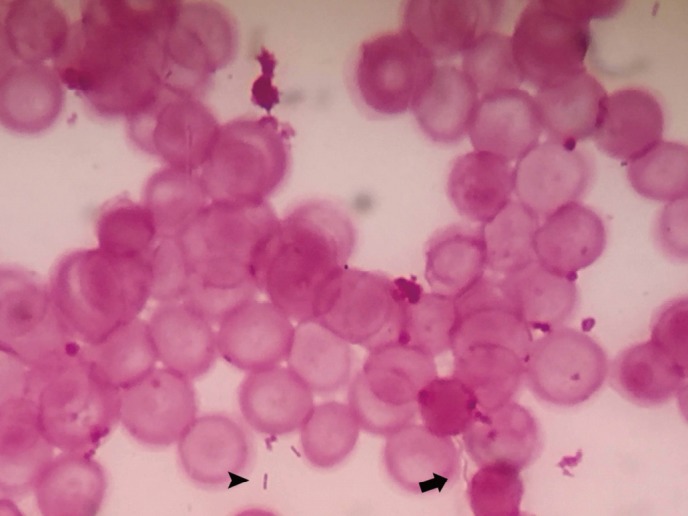
One of the anaerobic blood culture specimens revealed positivity for Bacteroides vulgatus after 53 hours. The other anaerobic blood culture specimen revealed positivity for gram-negative short bacilli and gram-negative spiral-shaped bacilli after 120 hours, which were identified by direct Gram staining (Fig. 1).
Isolation was performed using 5% sheep blood trypticase soy agar at 37℃ under CO2 for 96 hours; small white colonies and swarming film-like colonies were detected. Direct Gram staining showed gram-negative short bacilli in the small colonies and gram-negative spiral-shaped bacilli in the swarming colonies. We could not identify either strain, using the Vitek 2 (bioMérieux, Lyon, France) automated bacterial identification system. We then performed 16S rRNA sequencing with the primer pair 27F and 1492R and identified the short bacilli as B. virosa (1,388 bp; 98.34% similarity to B. virosa MT12(T) strain, accession number: LC259309, DNA Data Bank of Japan, http://getentry.ddbj.nig.ac.jp) and the spiral-shaped bacilli as B. pilosicoli (1,346 bp; 99.93% similarity to B. pilosicoli P43/6/78(T) strain, accession number: LC259310 using the Eztaxon database). These results were confirmed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis using the Bruker MALDI BioTyper system software version 3.1 library database: 5672 (Bruker Daltonik GmbH, Bremen, Germany); B. virosa: score value 1.876, B. pilosicoli: score value 2.039.
Only 12 previous reports described the isolation of B. pilosicoli from blood culture [7]. Eleven of these 12 isolates were identified by 16S rRNA sequencing. All of the patients in these studies had serious underlying diseases, and six patients died. Similarly, in our case, translocation of these bacteria from the intestinal tract into the bloodstream was thought to have occurred.
Butyricimonas spp., members of the phylum Bacteroidetes, are normal inhabitants of the intestine in humans and animals. Only three cases of human infections due to B. virosa have been reported [8], all of which were bacteremia cases and two of which were associated with gastrointestinal tumors. Our patient had a history of carcinoma of the stomach and appendix. A number of reports have demonstrated the association between intestinal spirochetosis and sessile serrated adenomas, polyps, and mucinous adenocarcinoma [9]. Sinha et al [10] described the correlation between fecal microbe-metabolite networks and colorectal cancer. Their study suggests that increased carriage of specific microorganisms such as Fusobacterium, Atopobium, and Porphyromonas (to which B. virosa belongs), as well as their metabolites, may be associated with colorectal cancer.
In conclusion, we report a case of polymicrobial bacteremia with B. pilosicoli and B. virosa due to peritonitis following intestinal perforation. Incubation for over three to five days and 16S rRNA sequencing or MALDI-TOF mass spectrometry were necessary to recover and identify these organisms.
References
1. Weinstein MP, Reller LB, Murphy JR. Clinical importance of polymicrobial bacteremia. Diagn Microbiol Infect Dis. 1986; 5:185–196. PMID: 3757473.
2. Lin JN, Lai CH, Chen YH, Chang LL, Lu PL, Tsai SS, et al. Characteristics and outcomes of polymicrobial bloodstream infections in the emergency department: a matched case-control study. Acad Emerg Med. 2010; 17:1072–1079. PMID: 21040108.
3. Lassmann B, Gustafson DR, Wood CM, Rosenblatt JE. Reemergence of anaerobic bacteremia. Clin Infect Dis. 2007; 44:895–900. PMID: 17342637.
4. De Keukeleire S, Wybo I, Naessens A, Echahidi F, Van der Beken M, Vandoorslaer K, et al. Anaerobic bacteraemia: a 10-year retrospective epidemiological survey. Anaerobe. 2016; 39:54–59. PMID: 26923749.
5. Lee EH, Degener JE, Welling GW, Veloo AC. Evaluation of the Vitek 2 ANC card for identification of clinical isolates of anaerobic bacteria. J Clin Microbiol. 2011; 49:1745–1749. PMID: 21411572.
6. Sakamoto M, Takagaki A, Matsumoto K, Kato Y, Goto K, Benno Y. Butyricimonas synergistica gen. nov., sp. nov. and Butyricimonas virosa sp. nov., butyric acid-producing bacteria in the family ‘Porphyromonadaceae’isolated from rat faeces. Int J Syst Evol Microbiol. 2009; 59:1748–1753. PMID: 19542124.
7. Prim N, Pericas R, Espanol M, Rivera A, Mirelis B, Coll P. Bloodstream infection due to Brachyspira pilosicoli in a patient with multiorgan failure. J Clin Microbiol. 2011; 49:3697–3699. PMID: 21832021.
8. Enemchukwu CU, Ben-Faras H, Gialanella P, Szymczak WA, Nosanchuk JD, Madaline TF. Butyricimonas virosa bacteremia and bowel disease: case report and review. New Microbes New Infect. 2016; 13:34–36. PMID: 27408738.
9. Akiyama S, Kikuchi D, Mitani T, Fujii T, Yamada A, Matsui A, et al. A case of mucinous adenocarcinoma in the setting of chronic colitis associated with intestinal spirochetosis and intestinal stricture. Medicine (Baltimore). 2015; 94:e493. PMID: 25634199.
10. Sinha R, Ahn J, Sapmson JN, Shi Jianxin, Yu G, Siong X, et al. Fecal microbiota, fecal metabolome, and colorectal cancer interrelations. PLos One. 2016; 11:e0152126. PMID: 27015276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download