This article has been
cited by other articles in ScienceCentral.
Abstract
Clinical interpretation of the test results for cortisol based on continuous reference intervals with appropriate partitions improves pediatric diagnosis; however, these values are available only for Caucasians. To develop the pediatric reference intervals for Chinese population, we examined the serum cortisol levels in 1,143 healthy Chinese children aged 4–18 years (566 boys and 577 girls), using an IMMULITE 2000 Immunoassay System (Siemens Healthcare GmbH). Phlebotomy was performed at 7–9 a.m. for 284 boys and 287 girls and at 1–3 p.m. for the others. They were divided into four age groups according to the Clinical and Laboratory Standards Institute guideline EP28-A3c, with the last group further stratified according to sampling time. Separate reference intervals of 49.6–323.7, 70.9–395.3, and 90.1–448.7 nmol/L were established for children aged 4–8, 9–12, and 13–15 years, respectively. Further, reference intervals of 118.2–464.7 and 71.4–446.7 nmol/L were established for morning and afternoon cortisol levels, respectively, in children aged 16–18 years. Further studies are necessary to transfer and validate these reference intervals in other analytical systems and pediatric populations, and to allow for broader applications.
Go to :

Keywords: Cortisol, Reference interval, Pediatrics, Chinese
Measurement of cortisol is important in endocrinology. Children are more vulnerable to stress because the hypothalamic-pituitary-adrenal (HPA) axis is still developing. Consequently, clinical interpretation of the test results for cortisol based on continuous reference intervals (RIs) with appropriate partitions, which adequately characterize the physiological development, improves pediatric diagnosis.
Several studies have indicated the diurnal activity pattern of the HPA system in healthy populations and the divergence of normal circulating cortisol levels by age, sex, especially during puberty and adolescence, race and ethnicity, platform of analysis, and so on [
12]. Large initiatives in Canada, Germany, Scandinavia, and the U.S. have already begun working towards addressing the need for reliable and accurate pediatric RIs; however, most of the results from these projects are not published thus far [
3].
There has been no relevant national project in China. Therefore, we investigated serum cortisol levels in healthy Chinese children to develop key covariate stratified RIs in accordance with the Clinical and Laboratory Standards Institute guideline EP28-A3c [
4]. It must be noted that the previous investigations involved a small number of participants from ethnicities other than Caucasians [
3].
Healthy children who visited the Second Hospital Affiliated to Wenzhou Medical University for annual periodic health examination were recruited for the study from May 2013 to April 2016. These visits were organized by the K-12 schools in Wenzhou, China. Participation required the completion of a short questionnaire and a written informed consent. Eligible subjects were selected based on the results of their health assessments and questionnaire findings. The selected participants had normal body mass index according to the corresponding age and sex, based on cutoffs recommended by the Group of China Obesity Task Force [
5]. They had never suffered from chronic illnesses, had no acute disease in the previous month, had not taken any prescribed medications and performance enhancing over-the-counter medications and had not received medical treatment over the previous two weeks. The study was approved by the Human Research Ethics Committee at our hospital and was in accordance with the principles specified in the World Medical Association Declaration of Helsinki.
A total of 1,143 subjects (566 boys and 577 girls) participated in this study. Phlebotomy was performed at 7–9 a.m. for 284 boys and 287 girls and at 1–3 p.m. for the rest of the children. All children were of pure Chinese origin between the ages of 4–18 years. All Tanner stages (TS) were appropriate for their age.
Venous blood samples were allowed to clot for 20 minutes at room temperature. The sera were separated from the cells, and serum cortisol was measured, using an IMMULITE 2000 Immunoassay System (Siemens Healthcare GmbH, Erlangen, Germany) under well-controlled conditions and according to the manufacturer's instructions. Of note, mass spectrometry, which is the standard method for cortisol measurement, was not used; this is one of the limitations of this study. The determination limit was 5.5 nmol/L. A good linearity ranged from the determination limit to 2,069 nmol/L. The intra- and inter-assay coefficients of variation were within 3.7% and 5.5%, with 2.9% at 107.1 nmol/L and 3.1% at 391.0 nmol/L, respectively. Data were log-transformed to improve the normality of their distribution if necessary. Differences between groups were analyzed by performing the Cochran and Cox test using the SAS version 9.3 (SAS Institute Inc, Cary, NC, USA), with significance set at P<0.05.
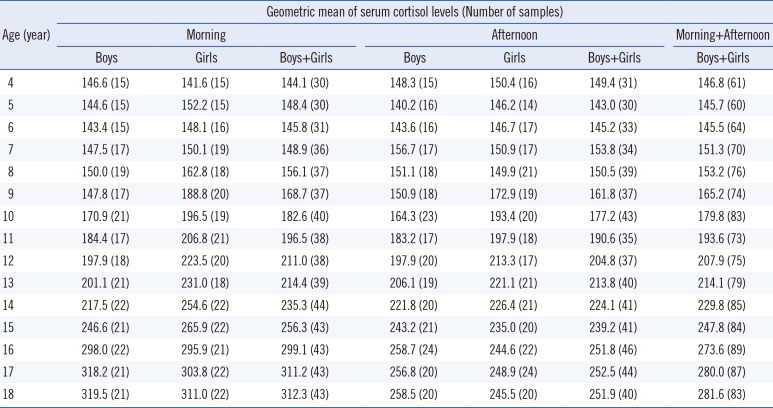
An apparent age effect on serum cortisol levels was noted, with cortisol levels increasing across the investigated age range as shown by the geometric means listed in
Table 1. This finding is similar to previous findings of Gunnar and Quevedo [
6] and Bailey et al [
7]; this was expected given that vast physiological changes occur during child development. Furthermore, log-transformed serum cortisol level positively correlated with TS: ln (serum cortisol level)=0.17×(TS)+4.76 (R
2=0.210,
P<0.001).
Table 1
Serum cortisol levels (nmol/L) in boys and girls of the same age (phlebotomy performed at 7–9 a.m. or 1–3 p.m.)
|
Age (year) |
Geometric mean of serum cortisol levels (Number of samples) |
|
Morning |
Afternoon |
Morning+Afternoon |
|
Boys |
Girls |
Boys+Girls |
Boys |
Girls |
Boys+Girls |
Boys+Girls |
|
4 |
146.6 (15) |
141.6 (15) |
144.1 (30) |
148.3 (15) |
150.4 (16) |
149.4 (31) |
146.8 (61) |
|
5 |
144.6 (15) |
152.2 (15) |
148.4 (30) |
140.2 (16) |
146.2 (14) |
143.0 (30) |
145.7 (60) |
|
6 |
143.4 (15) |
148.1 (16) |
145.8 (31) |
143.6 (16) |
146.7 (17) |
145.2 (33) |
145.5 (64) |
|
7 |
147.5 (17) |
150.1 (19) |
148.9 (36) |
156.7 (17) |
150.9 (17) |
153.8 (34) |
151.3 (70) |
|
8 |
150.0 (19) |
162.8 (18) |
156.1 (37) |
151.1 (18) |
149.9 (21) |
150.5 (39) |
153.2 (76) |
|
9 |
147.8 (17) |
188.8 (20) |
168.7 (37) |
150.9 (18) |
172.9 (19) |
161.8 (37) |
165.2 (74) |
|
10 |
170.9 (21) |
196.5 (19) |
182.6 (40) |
164.3 (23) |
193.4 (20) |
177.2 (43) |
179.8 (83) |
|
11 |
184.4 (17) |
206.8 (21) |
196.5 (38) |
183.2 (17) |
197.9 (18) |
190.6 (35) |
193.6 (73) |
|
12 |
197.9 (18) |
223.5 (20) |
211.0 (38) |
197.9 (20) |
213.3 (17) |
204.8 (37) |
207.9 (75) |
|
13 |
201.1 (21) |
231.0 (18) |
214.4 (39) |
206.1 (19) |
221.1 (21) |
213.8 (40) |
214.1 (79) |
|
14 |
217.5 (22) |
254.6 (22) |
235.3 (44) |
221.8 (20) |
226.4 (21) |
224.1 (41) |
229.8 (85) |
|
15 |
246.6 (21) |
265.9 (22) |
256.3 (43) |
243.2 (21) |
235.0 (20) |
239.2 (41) |
247.8 (84) |
|
16 |
298.0 (22) |
295.9 (21) |
299.1 (43) |
258.7 (24) |
244.6 (22) |
251.8 (46) |
273.6 (89) |
|
17 |
318.2 (21) |
303.8 (22) |
311.2 (43) |
256.8 (20) |
248.9 (24) |
252.5 (44) |
280.0 (87) |
|
18 |
319.5 (21) |
311.0 (22) |
312.3 (43) |
258.5 (20) |
245.5 (20) |
251.9 (40) |
281.6 (83) |

The differences in the morning or afternoon serum cortisol levels between boys and girls at the same age were not statistically significant, except for the morning cortisol levels at the age of 9 years (P=0.046). This was likely caused by early onset of puberty in girls compared to boys, who do not reach sexual development stage at this age. The geometric means of serum cortisol levels in both boys and girls who were eight years old and younger fluctuated at a low level. After this age, because of the puberty onset, both sexes experienced a dynamic elevation in cortisol levels, and the elevation was more dominant in girls than in boys. Therefore, pubertal girls' cortisol levels were higher than those of the age-matched boys. The serum cortisol levels in both sexes ascended to a plateau at the ages of 16–18 years, followed by a more dominant elevation in boys than in girls.
To the best of our knowledge, no investigations showed differences in cortisol levels between prepubertal boys and girls [
891011]. It has been proposed that sex related differences in HPA regulation emerge at puberty [
12], and there is ample literature supporting the divergence of cortisol levels during puberty and adolescence [
2]. However, the results of studies on cortisol levels in males and females at different pubertal stages are inconsistent. Netherton et al [
9] found that girls at TS 2 or more had 20–30% higher morning cortisol levels than their male counterparts. Scheifelbein and Susman [
13] reported greater cortisol levels in girls and in the later pubertal stages. Some investigations have suggested no sex related effect on cortisol levels at different pubertal stages [
8]. Bailey et al [
7] established identical age stratified RIs for boys and girls aged 0–19 years. In contrast, Tsai et al [
11] observed not only statistically significant sex related differences in the morning cortisol levels in subjects at TS 5 but also higher morning cortisol levels in boys than girls at the same stage.
When data from boys and girls of the same age were combined, the differences between morning and afternoon serum cortisol levels were not significant until the age of 16 years (
P=0.043, 0.031, and 0.018 for ages of 16, 17, and 18 years, respectively). The potential reasons for late occurrence of decreases in afternoon serum cortisol levels are as follows: first, it is a common phenomenon that phlebotomy causes stronger psychological stresses on younger children than on the grown-ups; second, the stresses may have proportionally increased the cortisol levels to a greater extent in samples obtained in the afternoon compared with those obtained in the morning, thereby minimizing the differences between morning and afternoon samples [
7].
It is generally assumed that as long as the difference between the observed means of the two subclasses is statistically significant, each subclass warrants its own RI [
4]. Hence, sex partitioning was unnecessary for our subject population. The Harris and Boyd test results recommended that the investigated age range be divided into four distinct groups (
Table 2). As expected, it was also recommended that 16–18-years-old children warrant their separate RIs for morning and afternoon serum cortisol levels.
Table 2
Harris and Boyd test results for partitioning of serum cortisol reference intervals

|
Groups to be partitioned |
z |
z*
|
|
4–8 years old vs 9–12 years old |
5.769 |
4.884 |
|
9–12 years old vs 13–15 years old |
5.646 |
4.554 |
|
13–15 years old vs 16–18 years old |
5.021 |
4.360 |
|
16–18 years old sampled in the morning vs in the afternoon |
4.011 |
3.116 |

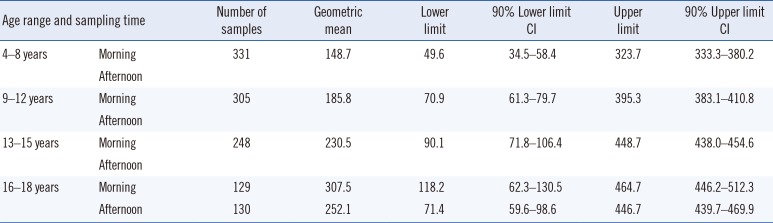
Age and sampling time stratified 95% RIs for serum cortisol and their 90% confidence intervals are shown in
Table 3. The levels for 9–12-years-old (
P=0.003), 13–15-years-old (
P<0.001), and 16–18-years-old (
P=0.020) groups were higher than those for the Caucasian dominated multiethnic Canadian counterparts, and the differences were statistically significant, except for the levels found in the 4–8-year-old age group (
P=0.180) [
7].
Table 3
Age and sampling time stratified pediatric 95% RIs for serum cortisol levels (nmol/L), their 90% confidence intervals, and geometric means analyzed with IMMULITE 2000
|
Age range and sampling time |
Number of samples |
Geometric mean |
Lower limit |
90% Lower limit CI |
Upper limit |
90% Upper limit CI |
|
4–8 years |
Morning |
331 |
148.7 |
49.6 |
34.5–58.4 |
323.7 |
333.3–380.2 |
|
Afternoon |
|
9–12 years |
Morning |
305 |
185.8 |
70.9 |
61.3–79.7 |
395.3 |
383.1–410.8 |
|
Afternoon |
|
13–15 years |
Morning |
248 |
230.5 |
90.1 |
71.8–106.4 |
448.7 |
438.0–454.6 |
|
Afternoon |
|
16–18 years |
Morning |
129 |
307.5 |
118.2 |
62.3–130.5 |
464.7 |
446.2–512.3 |
|
Afternoon |

Further studies are necessary to apply and validate these RIs in other analytical systems and other pediatric populations to allow for broader applications.







 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download