Abstract
The fecal immunochemical test (FIT) is the initial non-invasive investigation of choice for population-based colorectal cancer (CRC) screening. We evaluated the positivity rate in repeated tests using the same fecal specimen that showed borderline results in the FIT. A total of 6,465 patients were tested with the FIT in a tertiary-care hospital from July to December 2016. FIT was done using OC-Sensor PLEDIA (Eiken Chemical Co., Tokyo, Japan). Among 6,465 patients, 364 (5.6%) patients showed a positive FIT result of over 20 µg Hb/g feces. A total of 112 (1.7%) patients showed borderline scores of 10.2–20 µg Hb/g feces, and 5,989 (92.6%) patients showed negative results of less than 10 µg Hb/g feces. Among the 101 repeat-tested patients, 19 (18.8%) of the patients' scores converted to levels above the positive cut-off threshold. Repeated results of 19 patients showed score elevations from 20.2 to 68 µg Hb/g feces. These results suggest that it is most important to analyze properly prepared samples, even if only once. Therefore, the laboratory staff should ensure the proper preparation of stool specimens for FIT. Laboratory directors should choose the best cut-off value for detecting CRC at their respective institutions.
Colorectal cancer (CRC) is the second leading cause of cancer-related deaths in developed countries [1]. The fecal immunochemical test (FIT), which reveals hemoglobin in fecal occult blood, is the initial non-invasive investigation of choice for population-based CRC screening programs [234]. CRC has been included in the national cancer screening project in Korea since 2004, and FIT is conducted annually in adults over 50 years of age. A colonoscopy is recommended, if the FIT result is positive. Because the Korean government provides financial support for colonoscopies, physicians request a repeat test, if the FIT result is borderline. Furthermore, because the cut-off value can be adjusted according to medical scientific knowledge or the specifications of the screening program, the FIT cut-off value for CRC screening varies among institutions. We questioned whether a FIT value less than the cut-off value for CRC screening represents a true negative result. We evaluated the positivity rate for repeated tests using the same fecal specimen that showed borderline results in the FIT.
A total of 6,465 non-duplicated patients (men, 3,232; 50%), who were subjected to the FIT at Hanyang University Hospital, Seoul, Korea, from July to December 2016, were included in the analysis. The patients were individuals enrolled in the health check program (n=2,459, 38%), outpatients (n=2,908, 45%), and inpatients (n=1,098, 17%). The median age of the patients was 56 years (range, 0–95 years). A medical technician collected the fecal specimen and a single specimen per patient was used for FIT. Sample preparation was performed carefully by the same technician using bloody and mucoid stools. Repeated tests using the same specimens were immediately carried out for patients who showed borderline results, because the FIT result could be affected by influencing factors during storage and transportation [56]. FIT was performed using OC-Sensor PLEDIA (Eiken Chemical Co., Tokyo, Japan), which has been widely used in established screening programs [67]. An OC-Sensor test with a cut-off concentration of 20 µg Hb/g feces buffer was defined as positive, as determined by the Health Promotion Administration [8]. Because some institutions use a cut-off value of 10 µg Hb/g feces for CRC screening, we defined the borderline range as 10.2–20 µg Hb/g feces [910].
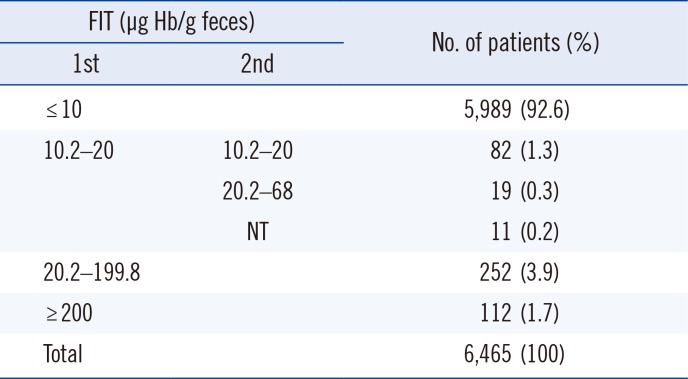
Among 6,465 patients, 364 (5.6%) were positive for the FIT with values over 20 µg Hb/g feces in the first test. A total of 112 (1.7%) patients showed borderline scores of 10.2–20 µg Hb/g feces, and 5,989 (92.6%) patients showed negative results of less than 10 µg Hb/g feces (Table 1).
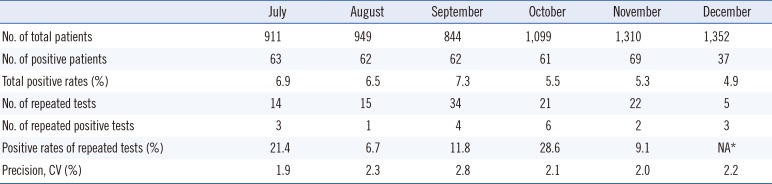
Table 2 shows the positivity rates and repeated positivity rates by month. The highest overall positivity rate (7.3%) was observed in September, and the lowest rate (4.9%) was observed in December. Among the repeated positivity tests for patients that showed borderline scores, the highest rate was 28.6% in October, and the lowest rate was 1.9% in July. Since only five of the patients with borderline scores were retested in December, the positivity rate could not be calculated in December since there were results for less than 10 patients (Table 2).
Among 101 repeat-tested patients, the scores of 19 (18.8%) patients converted to scores above the positive cut-off value. The repeated results of 19 patients showed score elevations from 20.2 to 68 µg Hb/g feces (Fig. 1). Among these, adenoma was found upon follow-up colonoscopy examination for one patient, and two patients had colonic adenoma. Four patients had chronic inflammation lesions in the colon, and one patient had an internal hemorrhoid. Two patients had unremarkable findings, and nine patients did not undergo colonoscopy.
A recent study emphasized that the FIT can identify patients that might receive the greatest benefit from colonoscopy, and a high rate of diagnostic colonoscopy was achieved after a positive FIT result [11]. However, a single FIT is insufficient for the detection of CRC or adenoma due to suboptimal sensitivity [1213]. In the Korean context, the lesion detection rates of the FIT were 21.39%, 42.53%, and 1.33% for the detection of adenoma, suspicious cancer lesion, and CRC, respectively [14]. However, a recent publication reported that using a two-sample FIT instead of a one-sample FIT did not result in a higher detection rate of advanced neoplasm [9]. They considered the results positive, if at least one sample was positive (cut-off of 10 µg Hb/g feces). After considering all of these factors, it appears to be most important to analyze properly prepared samples, even if only once. In addition, the results of the present study suggested that patients with borderline FIT results could benefit from a colonoscopy health check.
The limitations of this study included the followings: (1) we did not retest the positive specimens to check whether the results might change to negative; (2) the whole data set was not compared with the colonoscopy findings; (3) the definition of borderline is arbitrary. A follow-up study is necessary to examine borderline FIT results to improve the diagnostic efficiency of detecting CRC. Laboratory directors should consider the optimal cut-off value for detecting CRC at their respective institutions. In addition, the laboratory staff should be aware of carefully preparing stool specimens for FIT.
References
1. Lieberman D, Ladabaum U, Cruz-Correa M, Ginsburg C, Inadomi JM, Kim LS, et al. Screening for colorectal cancer and evolving issues for physicians and patients: a Review. JAMA. 2016; 316:2135–2145. PMID: 27893135.
2. Force USPST. Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW Jr, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2016; 315:2564–2575. PMID: 27304597.
3. Chiang TH, Chuang SL, Chen SL, Chiu HM, Yen AM, Chiu SY, et al. Difference in performance of fecal immunochemical tests with the same hemoglobin cutoff concentration in a nationwide colorectal cancer screening program. Gastroenterology. 2014; 147:1317–1326. PMID: 25200099.
4. Quintero E, Castells A, Bujanda L, Cubiella J, Salas D, Lanas A, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012; 366:697–706. PMID: 22356323.
5. Fraser CG, Allison JE, Young GP, Halloran SP, Seaman HE. Improving the reporting of evaluations of faecal immunochemical tests for haemoglobin: the FITTER standard and checklist. Eur J Cancer Prev. 2015; 24:24–26. PMID: 24584197.
6. Website: http://www.worldendo.org/wp-content/uploads/2016/08/weo_expert_working_group_fit_discussion_doc_no5_pu.pdf. 29 Aug 2017.
7. Passamonti B, Malaspina M, Fraser CG, Tintori B, Carlani A, D’Angelo V, et al. A comparative effectiveness trial of two faecal immunochemical tests for haemoglobin (FIT). Assessment of test performance and adherence in a single round of a population-based screening programme for colorectal cancer. Gut. 2016; 12. 14. pii: gutjnl-2016-312716. DOI: 10.1136/gutjnl-2016-312716. [Epub ahead of print].
8. Fraser CG, Allison JE, Halloran SP, Young GP. Expert working group on fecal immunochemical tests for hemoglobin CCSCWEO. A proposal to standardize reporting units for fecal immunochemical tests for hemoglobin. J Natl Cancer Inst. 2012; 104:810–814. PMID: 22472305.
9. Kapidzic A, van Roon AH, van Leerdam ME, van Vuuren AJ, van Ballegooijen M, Lansdorp-Vogelaar I, et al. Attendance and diagnostic yield of repeated two-sample faecal immunochemical test screening for colorectal cancer. Gut. 2017; 66:118–123. PMID: 26370109.
10. van Turenhout ST, van Rossum LG, Oort FA, Laheij RJ, van Rijn AF, Terhaar sive Droste JS, et al. Similar fecal immunochemical test results in screening and referral colorectal cancer. World J Gastroenterol. 2012; 18:5397–5403. PMID: 23082056.
11. Akram A, Gupta S. Fecal immunochemical testing: a sensitive and sustainable approach for population colorectal cancer screening? Gastroenterology. 2016; 151:554–555. PMID: 27477339.
12. Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008; 149:638–658. PMID: 18838718.
13. van Roon AH, Goede SL, van Ballegooijen M, van Vuuren AJ, Looman CW, Biermann K, et al. Random comparison of repeated faecal immunochemical testing at different intervals for population-based colorectal cancer screening. Gut. 2013; 62:409–415. PMID: 22387523.
14. Website: National Health Insurance Service Ilsan Hospital Research Report. 13 Jan 2017. Available at:http://lib.nhis.or.kr/search/detail/CATXAZ000000016556.
Fig. 1
First and repeated test results for fecal immunochemical test in 101 patients showing borderline scores.
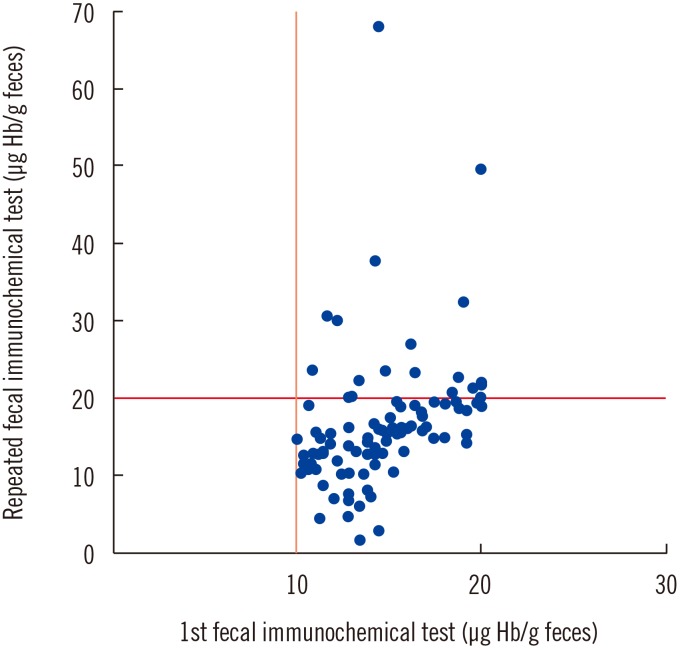
Table 1
Distribution of FIT results conducted over six months
| FIT (µg Hb/g feces) | No. of patients (%) | |
|---|---|---|
| 1st | 2nd | |
| ≤10 | 5,989 (92.6) | |
| 10.2–20 | 10.2–20 | 82 (1.3) |
| 20.2–68 | 19 (0.3) | |
| NT | 11 (0.2) | |
| 20.2–199.8 | 252 (3.9) | |
| ≥ 200 | 112 (1.7) | |
| Total | 6,465 (100) | |
Table 2
Monthly positive rates of fecal immunochemical tests in 2016




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download