Abstract
Traditional genetic counseling has focused on the target gene and its natural progress with respect to disease risk. Next-generation sequencing (NGS) can produce information on several genetic variants simultaneously, with different functions and consequences for each. Accordingly, determining the status of the patient or consultant and interpreting sequencing results from many genes can largely increase the complexity of genetic counseling. Moreover, the current environment of big data that can be readily shared via the internet and a ubiquitous network provides many different avenues for which a consultant must handle the traditional principle of genetic counseling in different ways. Thus, further consideration and rethinking of genetic counseling principles are necessary in the era of NGS. In this review, we discuss several aspects of genetic counseling that one can encounter when faced with NGS data.
Genetic counseling is the process by which patients or relatives at risk of developing a disorder with a potential hereditary component are advised of the consequences of the disorder, the probability of developing or transmitting it, and the ways in which the risk may be prevented, avoided, or ameliorated [1]. It is also defined as an educational process that seeks to assist affected and/or at-risk individuals to better understand the nature of a genetic disorder, its transmission, and the options open to them in management and family planning [1].
Traditionally, genetic counseling largely dealt with single-gene disorders or Mendelian diseases. However, with the progress of genomic technologies, a new era of multifactorial disease is on the horizon. Even a single-gene disorder is modified by the patient's background genetic make-up such as the presence of single nucleotide polymorphisms. For example, one study focused on the association of other genetic factors with ovarian cancer risk in BRCA1 and BRCA2 mutation carriers [2]. In addition, next-generation sequencing (NGS) technology enables analyses of multiple genes simultaneously, producing output on multiple variants, which makes the subsequent genetic counseling more demanding and complicated. This complexity arises because of the possibility of thousands of scenarios, which require different approaches to be assessed on a case-by-case basis.
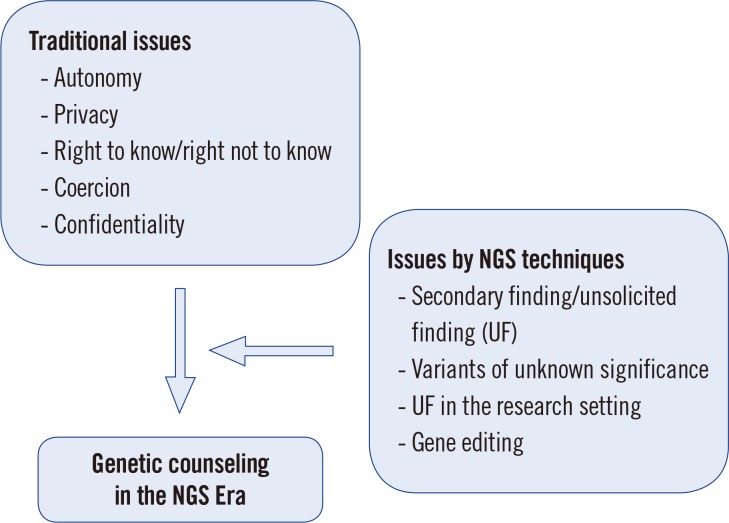
Within this context, it is essential to establish a general principle and new viewpoint for genetic counseling in the genomic age, as well as in the era of big data and ubiquitous networks (Fig. 1). In particular, application of genetic counseling should be specific for the context of the current culture of a society. Accordingly, the guidelines established for genetic counseling should vary depending on the cultural background. The Korean society has rapidly changed from an extended family to a nuclear family, thereby enhancing the concept of individualism; thus, these modern family dynamics should be considered in genetic counseling.
In this review, we address the general topics of genetic counseling with a focus on the specific considerations one might encounter when dealing with NGS data. Before starting this discussion, the terminology needs to be clarified. The term “incidental finding” is commonly used to refer to a finding not related to the primary diagnostic question. However, the American College of Medical Genetics (ACMG) has revised the term “incidental finding” to “secondary finding (SF)” because the designated genes to be studied are intentionally being analyzed as opposed to a genetic variant that is found incidentally [3]. By contrast, the European Society for Human Genetics (ESHG) prefers the term “unsolicited finding (UF)” [4]. In this article, both SF and UF will be used within the appropriate context.
The proband's/consultand's (P/C) medical and family history should be taken in a private room individually. Construction of a clear and detailed pedigree lays the foundation for good genetic counseling [1]. Only definite information should be used while constructing a pedigree. If possible, it is advisable to confirm the P/C's responses with objective evidence. It is important to always keep in mind that the P/C might provide some incorrect information during an interview, including details on family relationships. The same level of effort should be made with respect to gathering information for both the paternal and maternal sides of the family.
Besides molecular genetic tests, other laboratory tests such as biochemical tests are often included to provide complementary information during genetic counseling. It should be carefully considered whether or not to proceed with parental tests because these results can create a sense of guilt for the parents. Most of the relevant knowledge of a disease and its related genomics could be essentially gained through review of the literature; however, acquisition of information from websites or colleagues is equally important considering the rapid progress in the field. A mutation could be observed either directly in a patient or through indirect linkage analysis. When multiple variants of unknown significance (VUS) are found, indirect linkage analysis can help determine which VUS is more likely to be pathogenic. NGS, a revolutionary technology in genomics, consists of a gene panel, whole-exome sequencing (WES), or whole-genome sequencing (WGS) data. While performing NGS, it is important to check that the individual gene is covered and performance is adequate.
Diagnosis of a genetic disease and prenatal tests are defined by the Bioethics Law and Mother and Child Health Law in Korea (http://www.law.go.kr/lsInfoP.do?lsiSeq=188088&efYd=20170603#0000). Since there are sets of disorders for which tests can legally be conducted in Korea, one should check whether tests are legally permitted for the disease in question before performing the genetic test. Testing for late-onset disorders should normally be postponed until the individual can provide full informed consent [1].
Estimation of risks becomes much more important in the face of NGS data because multiple pathogenic variants or VUS could be obtained from one individual. To best explain the meaning of each variant to the P/C, the counselors should estimate the risks associated with the observed variants. For example, a VUS can be reclassified into other types of variants using different methods [56].
When the test result is first announced, it is very important to find a way to present the finding in a positive way to the P/C. In addition, since information on the mode of inheritance might create a feeling of guilt in the parents, a careful and prudent approach is needed while discussing this topic.
P/C autonomy refers to the P/C's right to make his/her own medical decision without considering the value judgment of others. However, individuals must first be deemed capable of making autonomous decisions in order to be awarded the rights of decision-making and privacy [7]. Non-autonomous individuals include minors (children), people with mental disability or illness, and prisoners. Persons in a certain age range, e.g., adolescents or younger children, could be considered to have some aspects of autonomy. Although respect for autonomy is an essential quality in genetic counseling, there are nevertheless some limitations [8]. For example, not all P/Cs have equal access to all medical choices owing to economic and social considerations. Indeed, autonomy is an ethical construct particular to Westernized cultures.
Uncritical dependence or overreliance on autonomy can result in a non-favorable outcome for patients and families. Familial autonomy may sometimes be more important than individual autonomy. Thus, although P/C autonomy is a fundamental principle of genetic counseling, this does not imply that autonomy should dominate over all other values in all situations. Rather, the concept of autonomy in genetic counseling should be applied in accordance with the cultural norms of a given society.
Another important concept to consider is non-directiveness that is a genetic counseling strategy, in which the genetic counselor tries to avoid influencing a P/C's decision [8]. “What would you do if you were in my place?” is the most commonly encountered question of a P/C during genetic counseling. The traditional and theoretical response is to avoid answering this question. However, such avoidance could make a P/C feel helpless and abandoned. Counselors can help the patient imagine the full extent of the consequences by explaining and providing examples based on rational and objective facts. By using this technique, the counselors engage in a process that can help promote patient decision-making.
The principle of privacy is also highly dependent on the cultural background, and thus varies from society to society or even from one group to another group in a single society. The Korean society is currently in a transitional phase, in which the value of privacy differs between elderly and young people. Elderly people tend to have no issues with sharing genetic test results among family members, whereas younger people might consider that disclosing one's genetic test result represents an infringement upon one's privacy, even when sharing among family members.
The concept of the “right not to know” in genetic counseling has been heavily debated [9101112]. Hofmann [13] insisted that as long as the information is not accurate and/or actionable, ignorance is bliss. According to several studies performed around the world, approximately 80% of individuals at risk for Huntington's disease did not want to know their carrier status [14]. Even for medically actionable diseases, some individuals feel comfortable not knowing their risk status [15]. Moreover, it is not technically simple to secure the right not to know with WES or WGS data, or even with a multiple gene panel test, as this would require a tremendous amount of time and effort to help the P/C decide what he/she wants to know and what he/she does not want to know among the large amount of data generated.
True coercion involves some type of harm or the threat of harm; however, there are many other types of more subtle pressure tactics possible, including persuasion and manipulation [7]. For example, the P/C might be persuaded by his/her relatives to undergo a genetic test. In this case, the counselor can provide other options such as offering a genetic test to other relatives.
Counselors need to respect client confidentiality by not releasing any client data to a third party, including the P/C's relatives [7]. Ethical dilemmas can occur when the P/C does not want to share genetic test results with relatives or wants to share genetic test results with some but not all relatives. However, some researchers have pointed out that the doctor's duty of confidentiality is not absolute [16]. In some very special circumstances, confidentiality may be overruled for the public interest or to prevent injury or severe health damage to other individuals [16].
In the United States, there is no single overarching legal authority dictating the requirements or limitations of prenatal testing or the clinical practices that surround how healthcare professionals offer screening or explain or treat genetic disorders [17]. By contrast, in Korea, the Bioethics Law and the Mother and Child Health Law permit prenatal test and preimplantation genetic diagnosis (PGD) only for particular sets of diseases, for e.g., testing for Duchenne muscular dystrophy is permitted, while that for Wilson's disease is not. We suggest that different criteria should be applied to prenatal diagnosis and PGD owing to the possibility of eugenics. This is an important consideration in terms of genetic diversity, because it is not known which genomes were present prior to survival in a particular environment. According to Sparrow [18], one could assert that it is not fair to compromise on the welfare of individuals in order to maintain diversity. We believe that society should come together in solidarity to support and protect individuals with diseases.
Gene editing methods such as clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 are complicated techniques that could be applied toward the creation of so-called “designer babies” toward eliminating disease-causing gene variants from the population; thus, this possibility should be urgently discussed in depth. Other issues related to gene editing should also be discussed based on the concept of social solidarity.
Whenever possible, children and adolescents should provide assent for testing and treatment [19]. There is no precise age at which an adolescent's wishes should be considered to be equally important as those of the parents. This decision will vary on a cultural, familial, legal, and individual basis. Upon reflection, adolescents of varying ages at the time of genetic counseling felt that they had a greater sense of stability when they were 15 to 16 years of age compared to the transition between elementary school and high school at age 13, which was associated with significant social adjustment and fears of not being accepted [20].
The ACMG has updated the recommendations for reporting of SF based on clinical genome sequencing data, which includes 59 medically actionable genes [3]. The ESHG has also recommended reporting only clinically actionable UF [21]. There is a wide spectrum of opinions regarding reporting VUS. The ACMG classified variants that should be reported as SF into known pathogenic and expected pathogenic variants, the latter of which being indicative of a “Sequence variation which is previously unreported and is of the type which is expected to cause the disorder” [22]. This recommendation classified genes for which expected pathogenic variants should or should not be reported. However, the ESHG has still not reached a consensus with regard to reporting VUS. With respect to the recommendation of the ACMG, there was no limitation placed on children when reporting SF. However, stricter rules are needed regarding children in societies with little solicitude for children, such as those in Korea.
Other aspects worthy of consideration include attitudes toward disease, as well as the values and culture of the society. Thus, in the Korean society, which is vulnerable to eugenics because of a fierce competitive environment, there is arguably greater importance in reporting UF or VUS. Hence, full discussion on reporting UF and VUS is absolutely necessary before application in practice. One strategy might be context-specific counseling based on the life stage.
Another aspect to consider is reporting UF in a research setting. Decisions about the disclosure of WGS and WES findings generated in a research setting are generally much more ethically contentious than decision-making in a clinical setting. Thus, Hallowell et al [23] called for greater transparency related to the purpose of sample collection, more explicit protocols for transitioning between research and clinical contexts, and more detailed warnings provided to patients and research participants of the potential for incidental findings to be generated, as well as their potential significance and actions that might be taken as a result.
A recent study assessed the preference for disclosure of SF results based on the responses of 200 families surveyed [15], which revealed various reactions to the topic. There may also be different opinions among different societies; thus, research on the preference for SF/UF should be conducted in the Korean population.
The Professional Society of Genetic Counselors in Asia recently provided an overview of the current status and challenges faced in 10 Asia-Pacific countries, and proposed a course of unified actions for the future of the genetic counseling profession [24].
In Korea, a certificate is given to non-medical personnel who have working experience in the field of genetic counseling. However, there are intrinsic ethical and legal problems in this system, because these non-medical personnel do not have knowledge of relevant medical ethics or a legal obligation of patient confidentiality. The current Korean medical law prohibits medical personnel from disclosing a patient's private information. In other words, non-medical personnel have no legal basis for obligations of patient confidentiality. Thus, it seems too early for non-medical personnel to perform genetic counseling. Moreover, genetic counseling requires a foundation of genomic knowledge besides a general knowledge of medicine, and there is good reason to doubt whether these non-medical personnel who have received training/education for only a limited time are suitably equipped to provide proper genetic counseling without detailed knowledge of both medicine and genomics.
NGS produces variants relevant to a range of diseases regardless of the traditional classification of medical specialties. Thus, the counselor should have comprehensive knowledge of both germline and somatic mutations, as well as of single gene disorders and multifactorial diseases.
The complex of genetic variants affecting the whole body and the specific medical problems presented by the P/C and family dynamics can become spiraling problems. Therefore, overall knowledge of general medicine is required in the NGS era more than ever. In this context, it seems that the traditional medical specialties cannot provide the appropriate services needed to correctly interpret the results derived from NGS data. Accordingly, even medical doctors should receive additional educational training for genomics, including NGS, in order to provide effective genetic counseling. Overall, there is clearly an urgent need to establish eligibility criteria for genetic counseling providers.
References
1. Harper P. Practical genetic counselling. 5th ed. Woburn, MA: Butterworth-Heinemann;1998. p. 3–4.
2. Vigorito E, Kuchenbaecker KB, Beesley J, Adlard J, Agnarsson BA, Andrulis IL, et al. Fine-scale mapping at 9p22.2 identifies candidate causal variants that modify ovarian cancer risk in BRCA1 and BRCA2 mutation carriers. PLoS One. 2016; 11:e0158801. PMID: 27463617.
3. Kalia SS, Adelman K, Bale SJ, Chung WK, Eng C, Evans JP, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med. 2017; 19:249–255. PMID: 27854360.
4. van El CG, Cornel MC, Borry P, Hastings RJ, Fellmann F, Hodgson SV, et al. Whole-genome sequencing in health care: recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2013; 21:580–584. PMID: 23676617.
5. Park KS, Cho EY, Nam SJ, Ki CS, Kim JW. Comparative analysis of BRCA1 and BRCA2 variants of uncertain significance in patients with breast cancer: a multifactorial probability-based model versus ACMG standards and guidelines for interpreting sequence variants. Genet Med. 2016; 18:1250–1257. PMID: 27124784.
6. Ryu JM, Kang G, Nam SJ, Kim SW, Yu J, Lee SK, et al. Suggestion of BRCA1 c.5339T>C (p.L1780P) variant confer from ‘unknown significance’ to ‘Likely pathogenic’ based on clinical evidence in Korea. Breast. 2017; 33:109–116. PMID: 28364669.
7. Schneider K. Counseling about cancer: strategies for genetic counseling. 3rd ed. New Jersey: Wiley-Blackwell;2012. p. 409–443.
8. Resta R. Complicated shadows: a critique of autonomy in genetic counseling. In : Leroy B, Veach P, Bartels DM, editors. Genetic counseling practice: advanced concepts and skills. New Jersey: Wiley-Blackwell;2010. p. 22.
9. Wilson J. To know or not to know? Genetic ignorance, autonomy and paternalism. Bioethics. 2005; 19:492–504. PMID: 16425486.
10. Andorno R. The right not to know: an autonomy based approach. J Med Ethics. 2004; 30:435–439. discussion 439-40. PMID: 15467071.
11. Ndinya-Achola J, Ambani J, Temmerman M, Piot P. The right not to know HIV-test results. Lancet. 1995; 345:969–970. PMID: 7619122.
12. Malpas P. The right to remain in ignorance about genetic information--can such a right be defended in the name of autonomy? N Z Med J. 2005; 118:U1611. PMID: 16132072.
13. Hofmann B. Incidental findings of uncertain significance: To know or not to know-that is not the question. BMC Med Ethics. 2016; 17:13. PMID: 26873084.
14. Robins Wahlin TB. To know or not to know: a review of behaviour and suicidal ideation in preclinical Huntington's disease. Patient Educ Couns. 2007; 65:279–287. PMID: 17000074.
15. Shahmirzadi L, Chao EC, Palmaer E, Parra MC, Tang S, Gonzalez KD. Patient decisions for disclosure of secondary findings among the first 200 individuals undergoing clinical diagnostic exome sequencing. Genet Med. 2014; 16:395–399. PMID: 24113345.
16. Husted J. Autonomy and a right not to know. In : Chadwick R, Levitt M, Shickle D, editors. The right to know and the right not to know. 2nd ed. Cambridge: Cambridge University Press;2014. p. 24–37.
17. Pergament D, Ilijic K. The legal past, present and future of prenatal genetic testing: professional liability and other legal challenges affecting patient access to services. J Clin Med. 2014; 3:1437–1465. PMID: 26237611.
18. Sparrow R. Imposing genetic diversity. Am J Bioeth. 2015; 15:2–10. PMID: 26030484.
19. Wertz DC, Fletcher JC, Berg K. Review of ethical issues in medical genetics. World Health Organization Human Genetics Programme. 2003. p. 62.
20. Pichini A, Shuman C, Sappleton K, Kaufman M, Chitayat D, Babul-Hirji R. Experience with genetic counseling: the adolescent perspective. J Genet Couns. 2016; 25:583–595. PMID: 26573304.
21. Hehir-Kwa JY, Claustres M, Hastings RJ, van Ravenswaaij-Arts C, Christenhusz G, Genuardi M, et al. Towards a European consensus for reporting incidental findings during clinical NGS testing. Eur J Hum Genet. 2015; 23:1601–1606. PMID: 26036857.
22. Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013; 15:565–574. PMID: 23788249.
23. Hallowell N, Hall A, Alberg C, Zimmern R. Revealing the results of wholegenome sequencing and whole-exome sequencing in research and clinical investigations: some ethical issues. J Med Ethics. 2015; 41:317–321. PMID: 25038088.
24. Laurino MY, Leppig KA, Abad PJ, Cham B, Chu YWY, Kejriwal S, et al. A report on ten Asia Pacific countries on current status and future directions of the genetic counseling profession: the establishment of the Professional Society of Genetic Counselors in Asia. J Genet Couns. 2017; 1–12.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download