Dear Editor,
Axenfeld-Rieger syndrome (ARS) is a rare autosomal dominant disorder characterized by ophthalmologic anterior segment abnormalities and extraocular findings, including dental anomalies, cardiovascular outflow tract malformations, and craniofacial abnormalities [1]. Mutations in paired like homeodomain 2 (PITX2) and forkhead box C1 (FOXC1) genes, which play important roles in embryonic development, are responsible for causing ARS [2]. Affected individuals with PITX2 mutations typically have systemic abnormalities in addition to ophthalmologic issues, while FOXC1 mutations are mainly associated with ocular lesions [3].
PITX2 is a member of the paired class of homeodomain transcription factors and affects the development of various anterior segment tissues that originate from the neural crest as well as several extraocular tissues such as the heart [4]. Missense, nonsense, and frameshift pathogenic variants of PITX2 have been identified in ARS [5]. These pathogenic variants are thought to affect the expression of PITX2.
In Korea, one family with PITX2-related ARS and two families with FOXC1-related ARS have been reported [678]. Here, we present the clinical findings of a Korean ARS family carrying a novel pathogenic PITX2 variant.
The proband (I-1) was a 46-year-old woman who had been treated for several years for glaucoma in the right eye. The patient had been blind in the left eye for the last 10 years and lacked a detailed medical history. At ophthalmologic examination, the patient showed corneal opacity in both eyes, with more severe presentation in the left eye. Visual acuity was 20/100 in the right eye, with no light perception in the left eye. Intraocular pressure (IOP) was 30 mmHg in the right eye. The right eye showed corneal endothelial decompensation, posterior embryotoxon, iris atrophy, and corectopia. She underwent corneal transplant for the right eye. One year later, she underwent glaucoma surgery because of uncontrolled IOP elevation. The patient had no systemic findings.
The 22-year-old second daughter (III-2) of the proband underwent ophthalmic examination because of her mother's ocular history. Visual acuity was 20/20 in both eyes. Anterior segment examination revealed posterior embryotoxon in both eyes, but no other abnormalities or glaucomatous optic nerve damage were found. Detailed glaucoma investigation, including visual field testing, revealed no abnormal signs. The patient showed microdontia, but no other systemic abnormalities were detected.
Under diagnostic suspicion of ARS, the proband's FOXC1 and PITX2 were sequenced after obtaining informed consent. Genomic DNA was isolated from peripheral blood leukocytes using the Wizard Genomic DNA Purification kit (Promega, Madison, WI, USA). All exons and their flanking intronic regions were amplified by polymerase chain reaction using primers designed by the authors (available on request), and Sanger sequencing was performed using an ABI Prism 3730xl Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). A novel heterozygous 2-bp deletion (c.475_476delCT) in PITX2, which was predicted to result in a frameshift and premature termination of PITX2 (p.Leu159Valfs*39), was detected. No pathogenic FOXC1 variants were detected. Similar genetic analysis of the second daughter confirmed inheritance of the c.475_476delCT (p.Leu159Valfs*39) variant (Fig. 1).
The c.475_476delCT variant was absent in dbSNP (build 149), Exome Aggregation Consortium (http://exac.broadinstitute.org/), and Korean reference genome databases at the time of the study (2017). This PITX2 c.475_476delCT variant can be considered a “pathogenic” variant according to the 2015 American College of Medical Genetics and Genomics and Association guidelines based on the following factors: (1) the variant is expected to cause premature termination (PVS1), (2) the variant is absent in large-population databases (PM2), and (3) patient phenotype is highly specific for the disease (PP4) [9].
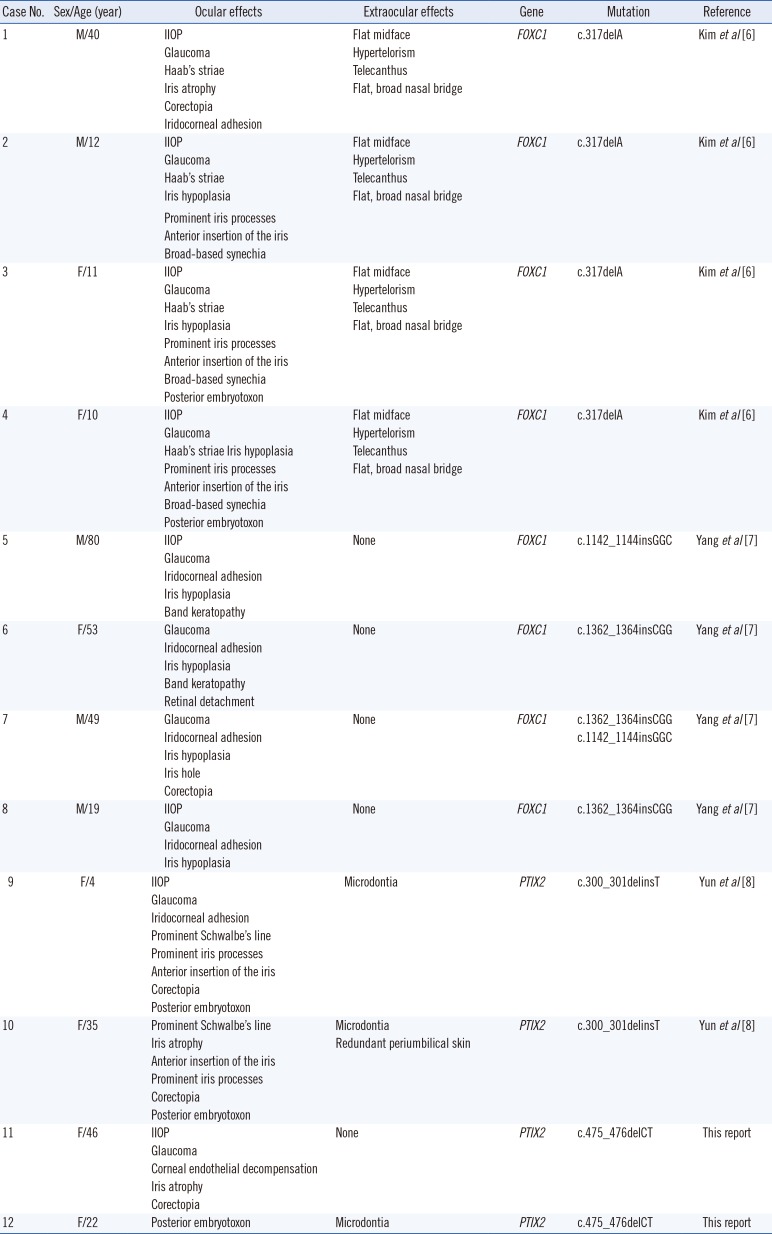
Ten cases of genetically confirmed ARS have been reported in Korea, in addition to the present two cases. The clinical features, including ocular and extraocular symptoms, and molecular features of these cases are described in Table 1.
Glaucoma is observed in approximately 50% of the ARS cases; however, for the proband's daughters (III-1, 2, and 3), no glaucomatous optic nerve damage was detected despite the presence of signs consistent with ARS [10]. The first daughter (III-1) showed microdontia without other systemic abnormalities or ocular signs. Visual acuity and IOP were in the normal range, and anterior segment examination and optic disc were normal. The second daughter (III-2) showed a typical ocular anterior segment finding of ARS, namely, posterior embryotoxon. However, no other ocular findings such as iris atrophy, corectopia, or glaucomatous optic nerve damage were present. Similar to her elder sister, she had microdontia, but no other systemic abnormalities. The third daughter (III-3) underwent ophthalmologic examinations at an outside clinic, but no abnormalities were detected. She had neither microdontia nor systemic abnormalities.
In conclusion, we identified a novel pathogenic PITX2 variant (c.475_476delCT;p.Leu159Valfs*39). This report will deepen our understanding of the genetic background of Korean ARS patients.
References
1. Chang TC, Summers CG, Schimmenti LA, Grajewski AL. Axenfeld-Rieger syndrome: new perspectives. Br J Ophthalmol. 2012; 96:318–322. PMID: 22199394.
2. Tumer Z, Bach-Holm D. Axenfeld-Rieger syndrome and spectrum of PITX2 and FOXC1 mutations. Eur J Hum Genet. 2009; 17:1527–1539. PMID: 19513095.
3. Hjalt TA. Current molecular understanding of Axenfeld-Rieger syndrome. Expert Rev Mol Med. 2005; 7:1–17.
4. Semina EV, Reiter R, Leysens NJ, Alward WL, Small KW, Datson NA, et al. Cloning and characterization of a novel bicoid-related homeobox transcription factor gene, RIEG, involved in Rieger syndrome. Nat Genet. 1996; 14:392–399. PMID: 8944018.
5. Reis LM, Tyler RC, Volkmann Kloss BA, Schilter KF, Levin AV, Lowry RB, et al. PITX2 and FOXC1 spectrum of mutations in ocular syndromes. Eur J Hum Genet. 2012; 20:1224–1233. PMID: 22569110.
6. Kim GN, Ki CS, Seo SW, Yoo JM, Han YS, Chung IY, et al. A novel forkhead box C1 gene mutation in a Korean family with Axenfeld-Rieger syndrome. Mol Vis. 2013; 19:935–943. PMID: 23687430.
7. Yang HJ, Lee YK, Joo CK, Moon JI, Mok JW, Park MH. A family with Axenfeld-Rieger syndrome: report of the clinical and genetic findings. Korean J Ophthalmol. 2015; 29:249–255. PMID: 26240509.
8. Yun JW, Cho HK, Oh SY, Ki CS, Kee C. Novel c.300_301delinsT mutation in PITX2 in a Korean family with Axenfeld-Rieger syndrome. Ann Lab Med. 2013; 33:360–363. PMID: 24003428.
9. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.
10. Allingham RR, Damji KF, Shields MB. Shields textbook of glaucoma. 6th ed. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins;2011. p. 227–235.
Fig. 1
Pedigree of the family with Axenfeld-Rieger syndrome (ARS) and results of PITX2 mutation analysis for the proband and proband's second daughter. (A) Open symbols indicate no signs or symptoms of ARS. Filled symbols represent affected individuals. Grey symbols indicate an individual who likely carries, but was not tested for, the PITX2 mutation. The arrow indicates the proband. (B) Sequencing analysis identified c.475_476delCT (p.Leu159Valfs*39) mutation.
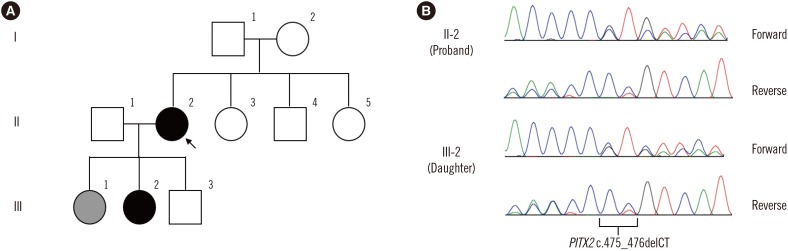
Table 1
Clinical features of genetically confirmed Axenfeld-Rieger syndrome patients in Korea
| Case No. | Sex/Age (year) | Ocular effects | Extraocular effects | Gene | Mutation | Reference |
|---|---|---|---|---|---|---|
| 1 | M/40 | IIOP | Flat midface | FOXC1 | c.317delA | Kim et al [6] |
| Glaucoma | Hypertelorism | |||||
| Haab's striae | Telecanthus | |||||
| Iris atrophy | Flat, broad nasal bridge | |||||
| Corectopia | ||||||
| Iridocorneal adhesion | ||||||
| 2 | M/12 | IIOP | Flat midface | FOXC1 | c.317delA | Kim et al [6] |
| Glaucoma | Hypertelorism | |||||
| Haab's striae | Telecanthus | |||||
| Iris hypoplasia | Flat, broad nasal bridge | |||||
| Prominent iris processes | ||||||
| Anterior insertion of the iris | ||||||
| Broad-based synechia | ||||||
| 3 | F/11 | IIOP | Flat midface | FOXC1 | c.317delA | Kim et al [6] |
| Glaucoma | Hypertelorism | |||||
| Haab's striae | Telecanthus | |||||
| Iris hypoplasia | Flat, broad nasal bridge | |||||
| Prominent iris processes | ||||||
| Anterior insertion of the iris | ||||||
| Broad-based synechia | ||||||
| Posterior embryotoxon | ||||||
| 4 | F/10 | IIOP | Flat midface | FOXC1 | c.317delA | Kim et al [6] |
| Glaucoma | Hypertelorism | |||||
| Haab's striae Iris hypoplasia | Telecanthus | |||||
| Prominent iris processes | Flat, broad nasal bridge | |||||
| Anterior insertion of the iris | ||||||
| Broad-based synechia | ||||||
| Posterior embryotoxon | ||||||
| 5 | M/80 | IIOP | None | FOXC1 | c.1142_1144insGGC | Yang et al [7] |
| Glaucoma | ||||||
| Iridocorneal adhesion | ||||||
| Iris hypoplasia | ||||||
| Band keratopathy | ||||||
| 6 | F/53 | Glaucoma | None | FOXC1 | c.1362_1364insCGG | Yang et al [7] |
| Iridocorneal adhesion | ||||||
| Iris hypoplasia | ||||||
| Band keratopathy | ||||||
| Retinal detachment | ||||||
| 7 | M/49 | Glaucoma | None | FOXC1 | c.1362_1364insCGG | Yang et al [7] |
| Iridocorneal adhesion | c.1142_1144insGGC | |||||
| Iris hypoplasia | ||||||
| Iris hole | ||||||
| Corectopia | ||||||
| 8 | M/19 | IIOP | None | FOXC1 | c.1362_1364insCGG | Yang et al [7] |
| Glaucoma | ||||||
| Iridocorneal adhesion | ||||||
| Iris hypoplasia | ||||||
| 9 | F/4 | IIOP | Microdontia | PTIX2 | c.300_301delinsT | Yun et al [8] |
| Glaucoma | ||||||
| Iridocorneal adhesion | ||||||
| Prominent Schwalbe's line | ||||||
| Prominent iris processes | ||||||
| Anterior insertion of the iris | ||||||
| Corectopia | ||||||
| Posterior embryotoxon | ||||||
| 10 | F/35 | Prominent Schwalbe's line | Microdontia | PTIX2 | c.300_301delinsT | Yun et al [8] |
| Iris atrophy | Redundant periumbilical skin | |||||
| Anterior insertion of the iris | ||||||
| Prominent iris processes | ||||||
| Corectopia | ||||||
| Posterior embryotoxon | ||||||
| 11 | F/46 | IIOP | None | PTIX2 | c.475_476delCT | This report |
| Glaucoma | ||||||
| Corneal endothelial decompensation | ||||||
| Iris atrophy | ||||||
| Corectopia | ||||||
| 12 | F/22 | Posterior embryotoxon | Microdontia | PTIX2 | c.475_476delCT | This report |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download