Dear Editor,
The increasing incidence of carbapenem-resistant Enterobacteriaceae (CRE) is a major concern for global health [1]. Among CRE, the prevalence of carbapenemase-producing Enterobacteriaceae (CPE) ranges from 11% to 20.1% in Korea [23]. The most clinically significant CPE are of the KPC-, IMP-, VIM-, NDM-, and OXA-48 types, mostly identified from Klebsiella pneumoniae isolates as sources of nosocomial outbreaks [4]. The microorganisms carrying these genes are a grave threat to global health, not only because of their resistance capacity but also because the genes are carried on plasmids. OXA-48-producing Enterobacteriaceae have been found worldwide since the first isolation of this gene from a K. pneumoniae isolate in Turkey in 2003 [4]. Although only one isolate of OXA-48-producing Escherichia coli has been previously detected in Korea in a urine specimen from a foreign patient [2], such bacteria are likely to emerge and spread owing to travel or patient exchanges from other countries [4]. NDM-1- and NDM-5-producing E. coli have been reported in Korea, although they are uncommon [56]. While NDM and OXA co-producing Enterobacteriaceae are emerging [47], NDM-5 and OXA-48 co-producing E. coli have not been reported worldwide to date. Here, we describe the first identification of NDM-5 and OXA-48 co-producing E. coli in Korea. This case has got an exemption (2017-11-0222) from the approval of the Institutional Review Board for Human Research in Yonsei University Wonju Severance Christian Hospital. Informed consent from the patient was not required for this report because the patient was de-identified.
A 76-year-old female patient was admitted to the Wonju Severance Christian Hospital for aortic valve replacement surgery due to severe aortic stenosis. The patient had a medical history of recent cerebral infarction, coronary artery occlusion disease, paroxysmal atrial fibrillation, and hypertension. She had no known recent history of travel. Following the surgery, she was treated for pneumonia with cefepime. A few days later, the patient newly developed a fever of 38.2℃. Two aerobic and anaerobic blood culture sets drawn from both arms were incubated in the BacT/Alert 3D system (bioMérieux, Marcy l'Etoile, France), and no organisms were detected from the blood cultures after five days of incubation. The laboratory findings showed an elevated white blood cell count of 11.22×109/L (segmented neutrophils, 67.2%) and serum C-reactive protein level of 224.76 nmol/L (reference range: <28.57 nmol/L). The platelet count decreased to 86×109/L. Urinalysis showed bacteriuria, and urine culture revealed the presence of Enterococcus faecium (>105 CFU/mL). Levofloxacin was administered to the patient, and the fever was reduced to 37.1℃. The patient was discharged with a follow-up urine culture. The urine specimen was inoculated onto 5% sheep blood agar (KOMED Life Science Co., Seongnam, Korea) and MacConkey agar plate.
After overnight incubation at 35℃, lactose-fermenting colonies (>105 CFU/mL) grew on the MacConkey agar and were identified as E. coli showing resistance to all tested antimicrobial agents except amikacin, tigecycline, and colistin, using VITEK 2 (bioMérieux) and MicroScan (Beckman Coulter, Brea, CA, USA) (Table 1). The probabilities of the presence of E. coli by VITEK 2 and MicroScan were 99% and 99.9%, respectively. To confirm species identification and antimicrobial susceptibilities, a modified Hodge test, carbapenemase inhibition test, XpertCarba-R assay (Cepheid, Sunnyvale, CA, USA), and REBA-EAC assay (a PCR-based reverse blot hybridization assay for detection of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases) were performed (Table 1) [89]. PCR-amplified products of the blaNDM and blaOXA-48-like genes were sequenced using in-house primers NDM-290F (5′-GTT GGT CGA TAC CGC CTG GAC CGA T-3′) and NDM-516R (5′-AGTCAGGCTGTGTTGCGCCGCAAC-3′), OXA48-80F (5′-GTT GGA ATG CTC ACT TTA CTG-3′) and OXA48-279R (5′-AAG ACT TGG TGT TCA TCC TTA AC-3′), respectively. Based on the results, the isolate was confirmed as harboring the blaCTX-M-1, blaACT, blaCMY, blaNDM-5, and blaOXA-48-like genes (Table 1).
Although the route of infection is unclear, this represents the first isolation of an blaNDM-5- and blaOXA-48-like-co-producing uropathogenic E. coli strain in Korea. Since the concurrent presence of other carbapenemase genes can lead to higher minimum inhibitory concentrations of carbapenems than an individual gene would, the emergence of this species should not be neglected [7]. In addition to the previously published CPE cases, this case demonstrates the importance of molecular identification of resistance mechanisms in carbapenem-resistant E. coli isolates to detect the emergence of such genes and of applying infection control measures to minimize their dissemination.
References
1. Sassi A, Loucif L, Gupta SK, Dekhil M, Chettibi H, Rolain JM. NDM-5 carbapenemase encoding gene in multidrug-resistant clinical isolates of Escherichia coli from Algeria. Antimicrob Agents Chemother. 2014; 58:5606–5608. PMID: 24982080.
2. Ahn S, Sung JY, Kim H, Kim MS, Hwang Y, Jong S, et al. Molecular epidemiology and characterization of carbapenemase-producing Enterobacteriaceae isolated at a university hospital in Korea during 4-year period. Ann Clin Microbiol. 2016; 19:39–47.
3. Jeong SH, Kim HS, Kim JS, Shin DH, Kim HS, Park MJ, et al. Prevalence and molecular characteristics of carbapenemase-producing Enterobacteriaceae from five hospitals in Korea. Ann Lab Med. 2016; 36:529–535. PMID: 27578505.
4. Dortet L, Poirel L, Al Yaqoubi F, Nordmann P. NDM-1, OXA-48 and OXA-181 carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. Clin Microbiol Infect. 2012; 18:E144–E148. PMID: 22404169.
5. Yoo JS, Kim HM, Koo HS, Yang JW, Yoo JI, Kim HS, et al. Nosocomial transmission of NDM-1-producing Escherichia coli ST101 in a Korean hospital. J Antimicrob Chemother. 2013; 68:2170–2172. PMID: 23696618.
6. Park M, Park SD, Lee MH, Kim SH, Lim K, Lee G, et al. The first report of NDM-5-producing uropathogenic Escherichia coli isolates in South Korea. Diagn Microbiol Infect Dis. 2016; 85:198–199. PMID: 27049587.
7. Khajuria A, Praharaj AK, Kumar M, Grover N. Emergence of Escherichia coli, co-producing NDM-1 and OXA-48 carbapenemases, in urinary isolates, at a tertiary care centre at Central India. J Clin Diagn Res. 2014; 8:DC01–DC04.
8. Song W, Yoo G, Hwang GY, Uh Y. Evaluation of diagnostic performance of RAPIDEC CARBA NP test for carbapenemase-producing Enterobacteriaceae. Ann Clin Microbiol. 2016; 19:59–64.
9. Wang HY, Yoo G, Kim J, Uh Y, Song W, Kim JB, et al. Development of a rapid reverse blot hybridization assay for detection of clinically relevant antibiotic resistance genes in blood cultures testing positive for gramnegative bacteria. Front Microbiol. 2017; 8:185. PMID: 28232823.
10. CLSI. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100-S27. 27th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2017.
Table 1
Results of antimicrobial susceptibility and carbapenemase producer tests on Escherichia coli isolate
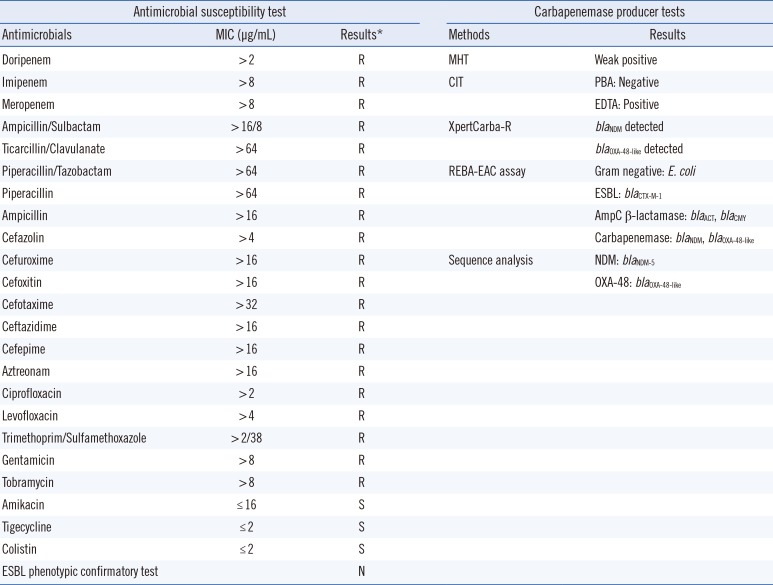
*Interpretative criteria of susceptibilities recommended by the Clinical and Laboratory Standards Institute guidelines [10].
Abbreviations: MIC, minimal inhibitory concentration; R, resistant; S, susceptible; N, negative; MHT, modified Hodge test; CIT, carbapenemase inhibition test; PBA, phenylboronic acid; EDTA, ethylenediaminetetraacetic acid; REBA-EAC, reverse blot hybridization assay for detection of extended-spectrum β-lactamases, AmpC β-lactamases, and carbapenemases; ESBL, extended spectrum β-lactamase.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download