Abstract
Identifying the trends in community-onset Acinetobacter baumannii complex isolation and diversity according to temperature could help provide insight into the behavior of the A. baumannii complex. We performed a retrospective analysis of A. baumannii complex (Acinetobacter baumannii, Acinetobacter nosocomialis, Acinetobacter pittii, and Acinetobacter calcoaceticus) isolates obtained from patients at a Korean community hospital from 2006 to 2015 with reference to seasonal temperatures. The incidence rates were compared between warm (June–September) and cold (November–March) months, defined as an average mean temperature ≥20℃ and ≤5℃, respectively. Incidence rate was calculated as the number of cases per month, converted to cases/105 admissions for healthcare-acquired isolates and cases/103 outpatients for community-onset isolates. Approximately 3,500 A. baumannii complex cases were identified, and 26.2% of them were community-onset cases. The median (interquartile range) number of community-onset A. baumannii complex cases was significantly higher (P=0.0002) in warm months at 13.8 (9.5–17.6) than in cold months at 10.1 (6.3–13.2). There was a strong correlation between community-onset A. baumannii complex cases and temperature (Pearson's r=0.6805, P=0.0149). Thus, we identified a seasonality pattern for community-onset A. baumannii complex colonization or infection, but not for healthcare-acquired cases.
It is generally considered that infections are more frequent in summer months; indeed, summer peaks in the incidences of gram-negative bacterial infections among hospitalized patients have been reported [123]. However, the seasonality of the Acinetobacter baumannii complex remains unclear. An unusual seasonal pattern of Acinetobacter calcoaceticus isolation was reported in the 1970s [4], and seasonality was not observed for multidrug-resistant (MDR) A. baumannii complex isolates [5]. In this study, we evaluated the trends of A. baumannii complex isolation in community-onset and healthcare-acquired colonization or infection cases in Korea according to seasonal and temperature variations. These data are expected to contribute to gaining a better understanding of the behavior of the A. baumannii complex.
We performed a retrospective analysis of A. baumannii complex isolates from patients at National Health Insurance Service (NHIS) Ilsan Hospital (742 beds), located in Goyang-si in Gyeonggi-do province, Korea, from 2006 to 2015. The study was approved by NHIS Ilsan Hospital Institutional Review Board (NHIMC 2017-07-011). Species identification and antimicrobial susceptibility tests were performed using the Microscan Walk-away plus system (Beckman Coulter, Inc., Brea, CA, USA) with MicroScan Neg Breakpoint Combo Type 44 (Siemens Healthcare Diagnostics Inc., West Sacramento, CA, USA). With this method, A. baumannii identification represents the A. baumannii complex, including A. baumannii, Acinetobacter nosocomialis, Acinetobacter pittii, and A. calcoaceticus, because these closely related species cannot be differentiated using biochemical methods. When multiple isolates were detected in the same patient, only the first isolate obtained within a 90-day period was included in the study, according to Fukuta et al's scheme [5]. Healthcare-acquired isolates were defined as those obtained after 48 hours of admission, whereas all others were considered to be community-onset cases according to the definition provided by Friedman et al [6]. To standardize the sample size of the two patient groups for effective analysis, the number of A. baumannii complex cases per month was converted to cases per 105 admissions for healthcare-acquired isolates and to cases per 103 outpatients for community-onset isolates.
Monthly average high temperatures were obtained from the National Weather Service Forecast Office (http://www.kma.go.kr/index.jsp), and data obtained at Munsan-si (the nearest observation site from the hospital location) were used. We defined June to September (average mean temperature ≥20℃) as warm months and November to March (average mean temperature ≤5℃) as cold months. The difference in incidence between warm and cold months was compared using an independent two-sample t-test, and correlations with temperature were evaluated by Pearson's correlation analysis. If the Pvalue was less than 0.05, the difference was considered statistically significant. SAS software version 9.2 was used for statistical analyses (SAS Institute, Cary, NC, USA).
During the 10-year period, 3,520 unique A. baumannii complex cases were recorded, including 922 (26.2%) community-onset isolates and 2,598 (73.8%) healthcare-acquired isolates. Fig. 1 shows the trends in the monthly incidence of community-onset and healthcare-acquired A. baumannii complex isolation over the 10-year period, with monthly average temperatures in the region superimposed. As can be seen, two groups showed different trends. When the number of cases in warm months (June to September) was compared to that in cold months (November to March), the median number (interquartile range) of community-onset A. baumannii complex cases was 13.8 (9.5–17.6) in the warm months and 10.1 (6.3–13.2) in the cold months, representing a significant difference (P=0.0002). By contrast, there was no difference in the median number (interquartile range) of healthcare-acquired cases between warm (9.5 [7.4–10.5]) and cold (9.5 [6.7–11.6]) months (P=0.6053). As shown in Fig. 2, there was a positive correlation between temperature and the incidence of community-onset A. baumannii complex (r=0.6805, P=0.0149), but not healthcare-acquired A. baumannii complex (r=−0.309, P=0.3285).
We identified seasonality for community-onset A. baumannii complex colonization or infection, whereas this trend was not observed for healthcare-acquired A. baumannii complex isolation, despite the large number of cases over the 10-year study period. A. baumannii is considered an important nosocomial pathogen [7], and its isolation (actually A. baumannii complex) has dramatically increased in Korean hospitals in recent years, especially in intensive care units [8]. In particular, the clonal spread of OXA-23-producing, carbapenem-resistant A. baumannii complex has caused many outbreaks in Korean hospitals [9]. Healthcare-acquired A. baumannii complex is known to be highly clonal, and thus this incidence rate may have been affected by the endemic nature of Korean hospitals. In contrast, community-onset cases are thought to be less clonal, suggesting more variable sources of isolation and the impact of natural seasonal variations.
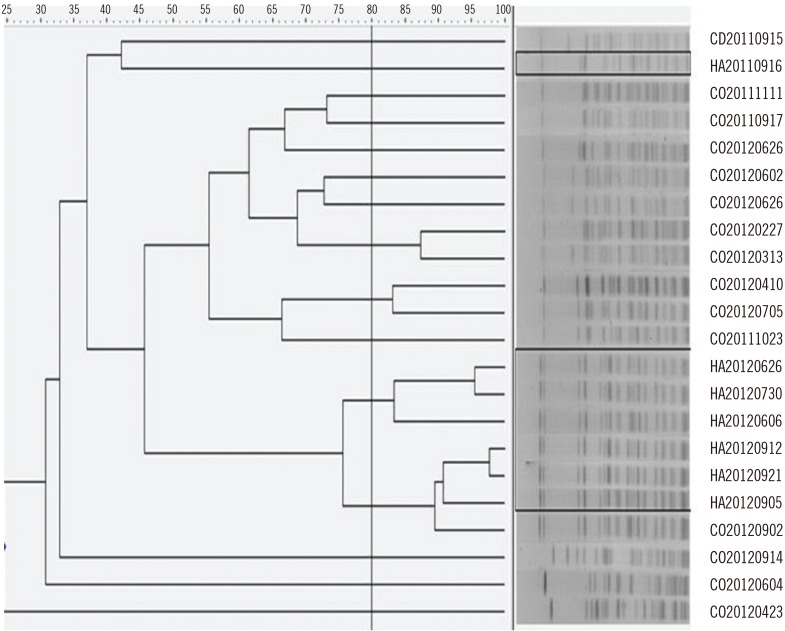
To further validate these results, we obtained pulsed-field gel electrophoresis profiles of SmaI-digested genomic DNA from 22 randomly selected A. baumannii isolates (including seven healthcare-acquired and 15 community-onset cases). With a cut-off threshold of 80% similarity, most of the healthcare-acquired isolates were clustered in similar groups, whereas the community-onset isolates showed a variety of band patterns (Fig. 3). Fukuta et al [5] also did not observe seasonality in the occurrence of the MDR A. baumannii complex in the United States, which likely reflects the highly clonal nature of this complex.
Although the specific reasons underlying the observed seasonal variation in A. baumannii complex isolation are not well elucidated, such seasonality has been primarily reported for gram-negative organisms [23]. Therefore, a possible explanation is that higher temperatures may promote the growth of bacteria in the environment and enhance the virulence of gram-negative bacteria, thereby contributing to increases in the infection incidence in warmer periods [1011]. Alternatively, seasonality might be related to the lipid A moiety of lipopolysaccharide, which forms the outer monolayer of the outer membrane of most gram-negative bacteria and is regulated by environmental conditions [11]. Recently, rapid-onset community-acquired pneumonia with a fulminant clinical course was reported in tropical regions [12], and extra-hospital reservoirs of A. baumannii complex are suspected as the cause [13]. A case of fulminant community-acquired A. baumannii complex pneumonia has already been reported in Korea [14]. Therefore, the emergence of community-associated A. baumannii complex infections should not be ignored, especially given climate change in Korea due to ongoing global warming [15].
There are some limitations in our study. This was a single-center study conducted in Gyeonggi province, and thus, further study is required in diverse clinical settings. The specific trends in A. baumannii isolation could not be precisely defined, because we used biochemical methods for species identification. Moreover, because recent exposure to healthcare facilities was not known for the cases defined as community-onset [6], it is possible that visits to other healthcare facilities prior to admission affected the calculated incidence of community-onset cases. Finally, we could not clarify whether the A. baumannii complex isolation represented a clinically relevant infection or colonization on the basis of clinical correlations.
In conclusion, we observed that the incidence of community-onset A. baumannii complex isolation is associated with seasonality and temperature fluctuation. This finding suggests that not only clonal spreading in healthcare facilities but also introduction from the community in warmer seasons could contribute to the dramatic increase in A. baumannii complex colonization or infection in Korea.
Acknowledgements
We acknowledge the work of the research and analysis team of NHIS Ilsan Hospital (Jung Hwa Hong and Hyunsun Lim, Biostatistics PhD).
References
1. Perencevich EN, McGregor JC, Shardell M, Furuno JP, Harris AD, Morris JG Jr, et al. Summer peaks in the incidences of gram-negative bacterial infection among hospitalized patients. Infect Control Hosp Epidemiol. 2008; 29:1124–1131. PMID: 19031546.
2. Eber MR, Shardell M, Schweizer ML, Laxminarayan R, Perencevich EN. Seasonal and temperature-associated increases in gram-negative bacterial bloodstream infections among hospitalized patients. PLoS One. 2011; 6:e25298. PMID: 21966489.
3. Richet H. Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect. 2012; 18:934–940. PMID: 22784293.
4. Retailliau HF, Hightower AW, Dixon RE, Allen JR. Acinetobacter calcoaceticus: a nosocomial pathogen with an unusual seasonal pattern. J Infect Dis. 1979; 139:371–375. PMID: 448188.
5. Fukuta Y, Clarke LG, Shields RK, Wagener MM, Pasculle AW, Doi Y. Lack of seasonality in the occurrence of multidrug-resistant Acinetobacter baumannii complex. Infect Control Hosp Epidemiol. 2012; 33:1051–1052. PMID: 22961027.
6. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 2002; 137:791–797. PMID: 12435215.
7. Lee K, Yong D, Jeong SH, Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011; 52:879–891. PMID: 22028150.
8. Lee Y, Kim YA, Song W, Lee H, Lee HS, Jang SJ, et al. Recent trends in antimicrobial resistance in intensive care units in Korea. Korean J Nosocomial Infect Control. 2014; 19:29–36.
9. Yang HY, Lee HJ, Suh JT, Lee KM. Outbreaks of imipenem resistant Acinetobacter baumannii producing OXA-23 beta-lactamase in a tertiary care hospital in Korea. Yonsei Med J. 2009; 50:764–770. PMID: 20046415.
10. Ratkowsky DA, Lowry RK, McMeekin TA, Stokes AN, Chandler RE. Model for bacterial culture growth rate throughout the entire biokinetic temperature range. J Bacteriol. 1983; 154:1222–1226. PMID: 6853443.
11. Raetz CR, Reynolds CM, Trent MS, Bishop RE. Lipid A modification systems in gram-negative bacteria. Annu Rev Biochem. 2007; 76:295–329. PMID: 17362200.
12. Dexter C, Murray GL, Paulsen IT, Peleg AY. Community-acquired Acinetobacter baumannii: clinical characteristics, epidemiology and pathogenesis. Expert Rev Anti Infect Ther. 2015; 13:567–573. PMID: 25850806.
13. Eveillard M, Kempf M, Belmonte O, Pailhoriès H, Joly-Guillou ML. Reservoirs of Acinetobacter baumannii outside the hospital and potential involvement in emerging human community-acquired infections. Int J Infect Dis. 2013; 17:e802–e805. PMID: 23672981.
14. Oh YJ, Song SH, Baik SH, Lee HH, Han IM, Oh DH. A case of fulminant community-acquired Acinetobacter baumannii pneumonia in Korea. Korean J Intern Med. 2013; 28:486–490. PMID: 23864808.
15. Center for Climate and Energy Solutions. South Korea and climate change. last accessed 16 June 2017. http://www.c2es.org/international/key-country-policies/south-korea.
Fig. 1
Incidence of Acinetobacter baumannii complex cases by calendar month, along with monthly average temperatures over 10-year study period.
*Number of cases were converted to cases per 103 outpatients (CO) or cases per 105 admissions (HA).
Abbreviations: CO, community-onset; HA, healthcare-acquired; Temp, temperature.
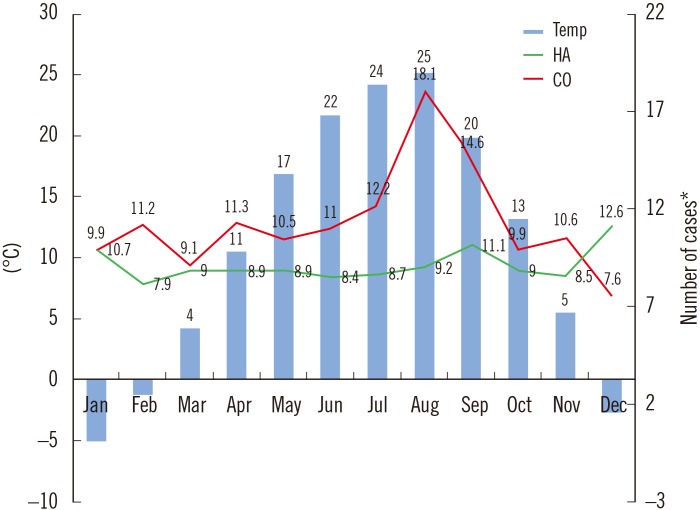
Fig. 2
Correlation of Acinetobacter baumannii complex cases by calendar month, along with monthly average temperatures from 2006 to 2015. (A) Temperatures (℃) vs Community-onset (cases/103 outpatients), y=2.69x-19.43, r=0.6805, P<0.05; (B) Temperatures (℃) vs Healthcare-acquired (cases/105 admissions) y=−2.45x+34.11, r=−0.309, P=0.3285 based on Pearson's correlation coefficient.
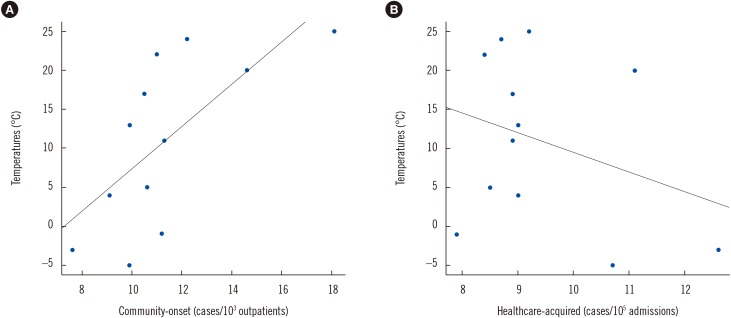
Fig. 3
PFGE profiles of SmaI-digested genomic DNA from isolates of Acinetobacter baumannii. The dashed line indicates 80% similarity. HA isolates are outlined, and the others are CO isolates. Numbers represent the date of specimen collection.
Abbreviations: PFGE, pulsed-field gel electrophoresis; HA, healthcare-acquired; CO, community-onset.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download