Dear Editor,
Lodderomyces elongisporus was first described as a cause of bloodstream infections in 2008 [1]. We present a case of L. elongisporus fungemia in a patient with lung cancer who had a central venous catheter.
A 56-year-old female presented at the emergency department of Asan Medical Center (Seoul, Korea) complaining of dyspnea. She had undergone a left lung lobectomy because of non-small cell lung cancer and had been treated with high-dose dexamethasone and cord decompression surgery for spinal cord metastasis two months ago; she was then treated with meropenem and teicoplanin for one week, owing to methicillin-resistant Staphylococcus aureus bacteremia. She was subsequently transferred to a secondary care hospital, in which a peripherally inserted central catheter had been placed for chemotherapy with docetaxel and antimicrobials. This study was performed retrospectively and was exempt from the requirements of informed consent and ethical approval.
Initial vital signs revealed a spiking fever up to 40℃. Abnormal laboratory findings included a peripheral leukocyte count of 19.9×109/L, platelets at 69×109/L, C-reactive protein at 1,937.2 nmol/L, lactate dehydrogenase at 6.13 µkat/L, prothrombin time of 14.2 seconds, activated partial thromboplastin time of 42.8 seconds, and D-dimer at 72.3 nmol/L. Chest computed tomography showed pleural effusion with multiple lung nodules and an edematous gall bladder.
Three sets of Bactec blood culture (Beckton-Dickenson, Sparks, MD, USA) collected at admission grew a single morphotype of yeast in aerobic vials only. Detection times were 25.7 hours for catheter-drawn blood and 28.6 and 31.1 hours in two peripheral blood sets. Microscopic evaluation of this yeast showed blastoconidia and branching pseudohyphae similar to those in Candida parapsilosis (Fig. 1).
CHROMagar Candida medium (Komed, Sungnam, Korea) revealed a turquoise-blue colony, which differed from the green colonies of Candida albicans and blue colonies of Candida tropicalis (Fig. 1). The Vitek 2 YST ID (bioMérieux, Durham, NC) and API 20C AUX (bioMérieux) system identified it as C. parapsilosis. However, Bruker Biotyper MALDI-TOF (Brucker Daltonics, Breman, Germany) analysis indicated a likely identification of L. elongisporus (score value=1.79), followed by C. parapsilosis (score value=1.12). Sequence analysis of an internal transcribed spacer (ITS) region of ribosomal DNA demonstrated 100% homology with L. elongisporus and 97% homology with C. parapsilosis.
By antifungal susceptibility testing using ATB Fungus 3 (bioMérieux), the isolate was determined to be susceptible to fluconazole, amphotericin B, voriconazole, micafungin, and caspofungin based on the CLSI breakpoint criteria for C. parapsilosis (Table 1) [2].
In the present case, L. elongisporus was identified as C. parapsilosis based on biochemical methods. Such misidentification is common because of the physiological similarities and phylogenetic relatedness between the species [1345]. As previously reported, ten isolates growing turquoise-blue colonies on CHROMagar were identified as C. parapsilosis biochemically, but as L. elongisporus by ITS sequencing [1]. Therefore, the distinct color on CHROMagar provides a clue [4]. MALDI-TOF is a reliable method to identify the C. parapsilosis complex, including L. elongisporus, to the species level.
This is the first report on human infection of L. elongisporus in Korea that was presented as catheter-related fungemia. It was considered a catheter-related bloodstream infection, because the patient had an indwelling central line catheter for one month and no other primary focus. L. elongisporus fungemia is rare and was only recently reported [45]. Only 15 cases have been reported to date, and two of the 15 cases were catheter-related [1345]. Indwelling catheter and the immunosuppressed status of this patient are attributed as risk factors of fungemia [5]. Large multigenic sequence analysis demonstrated that C. parapsilosis and L. elongisporus belong to the same clade, and rRNA gene sequencing confirmed their close relationship [5678]. C. parapsilosis is a well-known cause of catheter-associated fungemia via biofilm formation [6]. Therefore, L. elongisporus would be considered a potential causative agent of catheter-related fungemia.
The patient was septic and died three days after admission, before removal of the indwelling catheter or antifungal treatment. To date, three cases of L. elongisporus fungemia have been reported to be fatal due to underlying comorbidity [1345]. Other cases of L. elongisporus fungemia were generally susceptible to antifungal agents and often responded well to catheter removal and treatment with azoles or echinocandin [1345]. Invasive fungemia is associated with high mortality rates (>60%) when complicated with septic shock [9]. Prompt, empirical antifungal therapy could be recommended when a patient is septic despite antimicrobials and has a high rik of candidemia like this patient.
In conclusion, L. elongisporus infection may have been underestimated because of the possibility of misidentification. This case indicated that L. elongisporus fungemia could be fatal in septic condition.
References
1. Lockhart SR, Messer SA, Pfaller MA, Diekema DJ. Lodderomyces elongisporus masquerading as Candida parapsilosis as a cause of bloodstream infections. J Clin Microbiol. 2008; 46:374–376. PMID: 17959765.
2. CLSI. Reference method for broth dilution antifungal susceptibility testing of yeasts. 4th ed. Wayne, PA: Clinical and Laboratory Standards Institute;2012. CLSI supplement MM27-S4.
3. Ahmad S, Khan ZU, Johny M, Ashour NM, Al-Tourah WH, Joseph L, et al. Isolation of Lodderomyces elongisporus from the catheter tip of a fungemia patient in the Middle East. Case Rep Med. 2013; 2013:560406. PMID: 23653654.
4. Daveson KL, Woods ML. Lodderomyces elongisporus endocarditis in an intravenous drug user: a new entity in fungal endocarditis. J Med Microbiol. 2012; 61:1338–1340. PMID: 22683656.
5. Hatanaka S, Nakamura I, Fukushima S, Ohkusu K, Matsumoto T. Catheter-related bloodstream infection due to Lodderomyces elongisporus. Jpn J Infect Dis. 2016; 69:520–522. PMID: 26743142.
6. De Carolis E, Hensgens LA, Vella A, Posteraro B, Sanguinetti M, Senesi S, et al. Identification and typing of the Candida parapsilosis complex: MALDI-TOF MS vs. AFLP. Med Mycol. 2014; 52:123–130. PMID: 24577004.
7. James SA, Collins MD, Roberts IN. The genetic relationship of Lodderomyces elongisporus to other ascomycete yeast species as revealed by small-subunit rRNA gene sequences. Lett Appl Microbiol. 1994; 19:308–311. PMID: 7765443.
8. Tay ST, Na SL, Chong J. Molecular differentiation and antifungal susceptibilities of Candida parapsilosis isolated from patients with bloodstream infections. J Med Microbiol. 2009; 58:185–191. PMID: 19141735.
9. Delaloye J, Calandra T. Invasive candidiasis as a cause of sepsis in the critically-ill patient. Virulence. 2014; 5:161–169. PMID: 24157707.
Fig. 1
Culture and microscopic characteristics of Lodderomyces elongisporus isolated from the patient. CHROMagar medium showing green colonies for Candida albicans (A), turquoise-blue colonies for L. elongisporus (B), and blue colonies for Candida tropicalis (C). Microscopy of the colonies on Sabouraud dextrose agar revealed ellipsoidal to elongated blastoconidia of L. elongisporus (Gram stain, ×1,000) (D). Slide cultures on corn meal agar showed abundant branched pseudohyphae (Lactophenol cotton blue stain, ×400) (E).

Table 1
Antifungal susceptibility of Lodderomyces elongisporus isolated from the patient
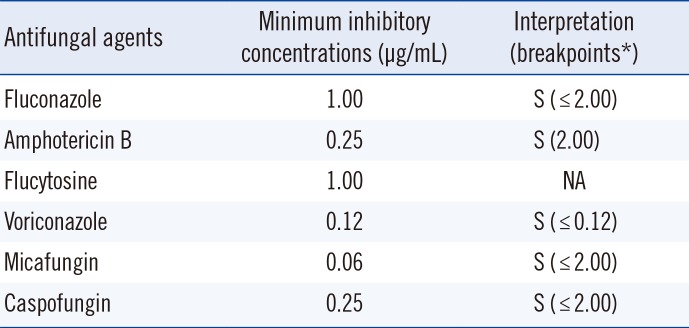
*Clinical and Laboratory Standards Institute breakpoints for Candida parapsilosis [2].
Abbreviations: NA, not applicable; S, susceptible; R, resistant.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download