Dear Editor,
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell disorder characterized by a reciprocal translocation between the long arms of chromosomes 9 and 22, resulting in the formation of a Philadelphia (Ph) chromosome. Most chimeric BCR-ABL1 fusion transcripts are e13a2(b2a2) or e14a2(b3a2), encoding a 210-kDa protein, p210, but some are a e1a2 transcript or e19a2 transcript encoding a 190-kDa protein or a 230-kDa protein, respectively [1]. Furthermore, some patients harbor rarer atypical transcript types such as e1a3, e6a2, e8a2, and e13a3. In addition, BCR-ABL1 transcripts have been reported to correlate significantly with the prognosis of CML patients. We retrospectively studied a CML patient with a variant BCR-ABL1 fusion transcript with no insertion between BCRexon8 and ABLexon2, to elucidate clinical characteristics of the rare e8a2 transcript CML.
The study was approved by the Human Research Ethics Committee of Wuhan Tongji hospital, Wuhan, China. Written informed consent was obtained from the patient. A 47-year-old woman-was referred to Tongji Hospital in December 2013. Peripheral blood analysis revealed a white blood cell count of 124×109/L (82.7% neutrophils, 7.2% lymphocytes, 5.9% monocytes, 3.0% eosinophils, and 1.2% basophils); platelet count of 613×109/L; and hemoglobin level of 10.8 g/dL. The patient showed typical characteristics indicative of splenomegaly. The bone marrow smear indicated an abnormal cell composition including 4% myeloblasts. G-banding karyotype analysis showed 46, XX, t (9;22)(q34;q11) in all 10 analyzed metaphase cells (Fig. 1A).FISH analysis revealed Ph-positive cells with one green BCR signal, one red ABL signal, and two yellow BCR-ABL fusion signals, which were present in 95% of 200 interphase cells counted (Fig. 1B). Nevertheless, we failed to detect typical BCR-ABL1 transcripts such as e13a2 or e14a2 by qualitative RT-PCR. Subsequently, Sanger sequencing of complementary DNA revealed the e8a2-type fusion transcript (Fig. 1C). Accordingly, the patient was diagnosed as having CML in the chronic phase.Therefore, imported imatinib (400 mg, po, qd) was administered. After two weeks, the imported imatinib was replaced by domestic imatinib (400 mg, po, qd) because of the high cost of the former. At the request of the patient, the cytogenetic response was measured irregularly using FISH rather than by performing molecular monitoring using real-time quantitative PCR (RTqPCR). Positive cells were undetectable after 17 months of treatment (Fig. 1D).
It is widely acknowledged that the direct junction between BCR exon e8 and ABL exon e2 fails to encode the chimeric oncogenic protein owing to the presence of a premature stop codon (UAG) at position 7 after the fusion locus [23]. Nevertheless, insertion of certain sequences could lead to the generation of an in-frame e8a2BCR-ABL1 transcript. In the 14 cases reported previously, insertion sequences from ABL intron 1b were identified in five cases; those from ABL intron 1a in three cases; those from BCR intron 8 in two cases; and those from another definite gene such as PRDM12, SPECC1L, or MAST2, in four cases [456]. Interestingly, in our case, sequencing analysis revealed that the BCR-ABL1 transcript was derived from a direct combination of BCR exon e8 to ABL exon e2, without any insertion between them. Moreover, this junction subtype of could indeed encode the oncogenic protein resulting in occurrence of CML. Furthermore, the number of bases in this insertion sequence contained three multiples of triplet bases [7]. Perhaps in simply considering the advent of the stop codon after the fusion contributing to the halt of normal translation of the BCR-ABL1 transcript, the previous reports misinterpreted the accurate coding sequence in the open reading frame of the fusion transcript.
According to the analysis of all cases, thrombocytosis could not predict the prognosis of patients. The insertion segment derived from particular functional genes including PRDM12, SPECC1L, or MAST2 demonstrated no association with poor molecular response. Additionally, tyrosine kinase inhibitors (TKIs) could effectively treat CML patients with the e8a2 transcript (Table 1). Just as in our case, the patient achieved complete cytogenetic response. Moreover, in an unique reported case where imatinib treatment was withdrawn after four years of constant complete molecular remission, the e8a2 transcript became detectable after three months and reverted to an undetectable status again four months after resumption of imatinib treatment.
It is recommended that RT-qPCR be utilized to monitor the molecular level precisely. TKIs have been demonstrated to be effective in the treatment of e8a2-positive patients. However, a larger number of CML patients with the e8a2 transcript are needed to study the clinical features and prognosis.
Acknowledgments
This study was supported by a grant from the National Natural Science Foundation of China (Grant Number-81272422).
References
1. Jain P, Kantarjian H, Patel KP, Gonzalez GN, Luthra R, KanagalShamanna R, et al. Impact of BCR-ABL transcript type on outcome in patients with chronic-phase CML treated with tyrosine kinase inhibitors. Blood. 2016; 127:1269–1275. PMID: 26729897.
2. Demehri S, Paschka P, Schultheis B, Lange T, Koizumi T, Sugimoto T, et al. e8a2 BCR-ABL: more frequent than other atypical BCR-ABL variants? Leukemia. 2005; 19:681–684. PMID: 15703785.
3. Tchirkov A, Couderc JL, Périssel B, Goumy C, Regnier A, Uhrhammer N, et al. Major molecular response to imatinib in a patient with chronic myeloid leukemia expressing a novel form of e8a2 BCR-ABL transcript. Leukemia. 2006; 20:167–168. PMID: 16270035.
4. Riva E, Manrique Arechavaleta G, De Almeida C, Costa V, Fernandez Del Campo M, Ifran González S, et al. A novel e8a2 BCR-ABL1 fusion with insertion of MAST2 exon 2 in a four-way translocation t (1;17;9;22) (p35;q24;q44;q11) in a patient with chronic myeloid leukemia. Leuk Lymphoma. 2016; 57:203–205. PMID: 25899399.
5. Reid AG, Nacheva EP. A potential role for PRDM12 in the pathogenesis of chronic myeloid leukaemia with derivative chromosome 9 deletion. Leukemia. 2004; 18:178–180. PMID: 14523459.
6. Huet S, Dulucq S, Chauveau A, Ménard A, Chomel JC, Maisonneuve H, et al. Molecular characterization and follow-up of five CML patients with new BCR-ABL1 fusion transcripts. Genes Chromosomes Cancer. 2015; 54:595–605. PMID: 26252834.
7. Chen L, Wu Y, You Y, Xiao M, Yao Y, Li W. A novel e8a2 BCR-ABL1 intronic fusion through insertion of a chromosome 22 BCR gene fragment into chromosome 9 in an atypical Philadelphia (Ph) chromosome chronic myeloid leukemia patient. Leuk Lymphoma. 2016; 57:2930–2933. PMID: 27118564.
8. Cayuela JM, Rousselot P, Nicolini F, Espinouse D, Ollagnier C, Bui-Thi MH, et al. Identification of a rare e8a2BCR-ABL fusion gene in three novel chronic myeloid leukemia patients treated with imatinib. Leukemia. 2005; 19:2234–2236.
9. Qin YZ, Jiang B, Jiang Q, Zhang Y, Jiang H, Li JL, et al. Imatinib mesylate resistance in a chronic myeloid leukemia patient with a novel e8a2 BCR-ABL transcript variant. Acta Haematol. 2008; 120:146–149. PMID: 19039205.
10. Park IJ, Lim YA, Lee WG, Park JS, Kim HC, Lee HJ, et al. A case of chronic myelogenous leukemia with e8a2 fusion transcript. Cancer Genet Cytogenet. 2008; 185:106–108. PMID: 18722880.
Fig. 1
The diagnosis and monitoring of CML. (A) Conventional karyotype analysis by G-banding showing typical translocation between chromosome 9 and 22 (arrows). (B) FISH analysis using BCR-ABL dual fusion probe. BCR-ABL-positive cells (red arrow), normal cells (green arrow). (C) Sanger sequencing of the nested RT-PCR product demonstrating the direct junction between BCR exon e8 and ABL exon a2 (reference sequence: BCR, NM_004327.3; ABL1, NM_005157.5). (D) Clinical response to treatment monitored by FISH.
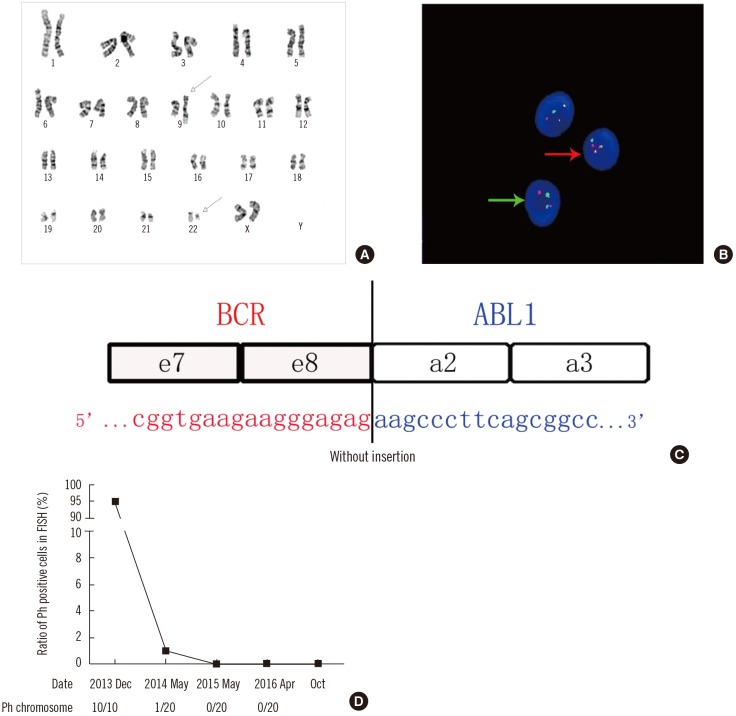
Table 1
Summary of CML cases with e8a2 BCR/ABL1 fusion transcript
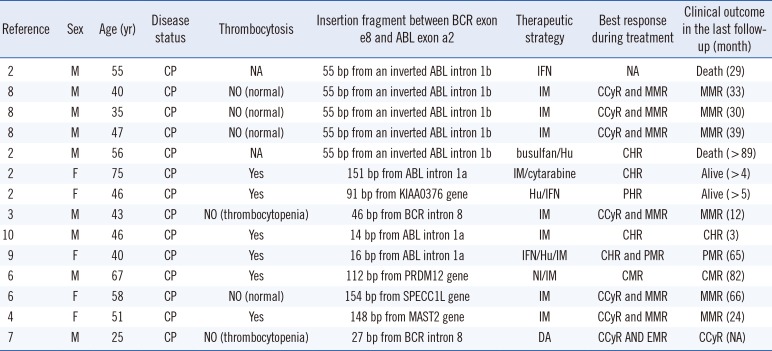
| Reference | Sex | Age (yr) | Disease status | Thrombocytosis | Insertion fragment between BCR exon e8 and ABL exon a2 | Therapeutic strategy | Best response during treatment | Clinical outcome in the last follow-up (month) |
|---|---|---|---|---|---|---|---|---|
| 2 | M | 55 | CP | NA | 55 bp from an inverted ABL intron 1b | IFN | NA | Death (29) |
| 8 | M | 40 | CP | NO (normal) | 55 bp from an inverted ABL intron 1b | IM | CCyR and MMR | MMR (33) |
| 8 | M | 35 | CP | NO (normal) | 55 bp from an inverted ABL intron 1b | IM | CCyR and MMR | MMR (30) |
| 8 | M | 47 | CP | NO (normal) | 55 bp from an inverted ABL intron 1b | IM | CCyR and MMR | MMR (39) |
| 2 | M | 56 | CP | NA | 55 bp from an inverted ABL intron 1b | busulfan/Hu | CHR | Death (> 89) |
| 2 | F | 75 | CP | Yes | 151 bp from ABL intron 1a | IM/cytarabine | CHR | Alive (> 4) |
| 2 | F | 46 | CP | Yes | 91 bp from KIAA0376 gene | Hu/IFN | PHR | Alive (> 5) |
| 3 | M | 43 | CP | NO (thrombocytopenia) | 46 bp from BCR intron 8 | IM | CCyR and MMR | MMR (12) |
| 10 | M | 46 | CP | Yes | 14 bp from ABL intron 1a | IM | CHR | CHR (3) |
| 9 | F | 40 | CP | Yes | 16 bp from ABL intron 1a | IFN/Hu/IM | CHR and PMR | PMR (65) |
| 6 | M | 67 | CP | Yes | 112 bp from PRDM12 gene | NI/IM | CMR | CMR (82) |
| 6 | F | 58 | CP | NO (normal) | 154 bp from SPECC1L gene | IM | CCyR and MMR | MMR (66) |
| 4 | F | 51 | CP | Yes | 148 bp from MAST2 gene | IM | CCyR and MMR | MMR (24) |
| 7 | M | 25 | CP | NO (thrombocytopenia) | 27 bp from BCR intron 8 | DA | CCyR AND EMR | CCyR (NA) |
Abbreviations: M, male; F, female; CP, chronic phase; IFN, interferon-alpha; Hu, hydroxyurea; IM, imatinib; DA, dasatinib; CHR, complete hematological response; CCyR, complete cytogenetic response; PMR, partial molecular response; MMR, major molecular response; CMR, complete molecular response; NA, not available.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download