Abstract
Guidelines recommend that clinical laboratories perform phenotypic tests (nitrocefin-based test and penicillin 10-U [P10] or 1-U [P1] zone edge tests) to detect penicillinase in Staphylococcus aureus isolates. This study aimed to assess the prevalence of blaZ encoding penicillinase and perform various phenotypic tests in S. aureus isolates from Japan. We prospectively collected 200 methicillin-susceptible S. aureus isolates from June 2015 to January 2016 and performed six phenotypic tests (nitrocefin-based test, P10 zone edge test/P10 diffusion test, penicillin 2-U [P2] zone edge test/P2 diffusion test, and cloverleaf test) on each sample. We confirmed the presence of blaZ (two blaZ-positive isolates) using PCR. Using blaZ PCR as a standard, we observed a low sensitivity (50%) and positive predictive value (PPV, 50%) of the nitrocefin-based test, low PPV (18.2%) of the P10 zone edge test, low sensitivity (50%) of the P10 diffusion test, low PPV (50% and 22.2%) of the P2 zone edge test and P2 diffusion test, respectively, and low sensitivity (50%) of the cloverleaf test. These data suggest a low performance (sensitivity and PPV) of these six phenotypic tests because of the low prevalence (1%) of blaZ in S. aureus isolates from Japan.
Some Staphylococcus aureus strains remain susceptible to penicillin G (Pc), although resistance rapidly emerged after introducing Pc. According to the Japan Nosocomial Infections Surveillance report in 2015 (http://www.nih-janis.jp/), 43.8% of 119,343 methicillin-susceptible S. aureus (MSSA) isolates from all registered medical institutes were susceptible to Pc. Thus, this antimicrobial agent remains the treatment of choice for patients infected with Pc-susceptible isolates, as Pc is considered superior to oxacillin against penicillinase-negative isolates. Reliable detection of penicillinase production is important, but the detection and reporting of Pc susceptibility and resistance remains difficult.
Two mechanisms contribute to Pc resistance in S. aureus; first, involving the production of penicillinase encoded by blaZ, which can inactivate Pc by hydrolyzing the β-lactam ring [1], and second, involving an altered Pc-binding protein, PBP2a, encoded by mecA [23]. blaZ is an 846-bp gene controlled by two regulatory genes (antirepressor blaR1 and repressor blaI) [3]. After exposure to β-lactams, blaR1 (transmembrane sensor–transducer) undergoes autocatalytic cleavage, promoting the cleavage of blaI and leading to the transcription of blaZ [14]. Serotype analysis has reported four types (Ambler class A) of penicillinase: A, C, and D are located on plasmids, while B is located on the chromosome [56].
Guidelines of the CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST) recommend that microbiological laboratories perform phenotypic tests (nitrocefin-based test and Pc 10-U [P10] or 1-U [P1] zone edge tests) to detect penicillinase in S. aureus strains [78]. Nitrocefin-based tests are reportedly less sensitive than Pc-disc zone edge determinations [91011]. This study aimed to assess the prevalence of blaZ and performance of various phenotypic tests using S. aureus isolates from Japan.
We prospectively collected and randomly selected non-duplicate 200 MSSA isolates with Pc minimum inhibitory concentrations (MICs) of ≤0.12 µg/mL (breakpoint judged as susceptible to Pc) based on the CLSI broth microdilution (BMD) method with the MicroScan WalkAway System (Beckman Coulter Inc., Tokyo, Japan) at one laboratory (Byotai-Seiri Laboratory), from June 2015 through January 2016 [7]. Patient information (including gender, age, and clinical specimens) was recorded. We performed six phenotypic tests (nitrocefin-based test, P10 zone edge test/P10 diffusion test, Pc 2-U [P2] zone edge test/P2 diffusion test, and cloverleaf test) for each screened isolate. P2 was applied as the minimum U of Pc because the recommended P1 was not available in Japan. Briefly, a BBL Cefinase Paper disc (Becton, Dickinson and Company, Tokyo, Japan) was used in the nitrocefin-based test [7]. This test was conducted using inoculum directly from the margin zone surrounding a cefoxitin disc (30 µg) placed on Mueller-Hinton agar (MHA) after 16–18 hours of incubation to induce penicillinase production in the isolates. When the disc showed a pink color at room temperature (approximately 20℃) within 1 hour of the reaction, the isolate was considered penicillinase-positive. Two different disc diffusion tests were performed on MHA by applying both P10 (BD Sensi-Disc, Becton, Dickinson and Company) and P2 (SP Check, Nissui Pharmaceutical Co., Ltd., Tokyo, Japan). P10 zone diameters were interpreted according to the CLSI criteria (isolates with a P10 diameter of ≤28 mm were considered resistant) [7], and P2 zone diameters were interpreted according to the EUCAST criteria (isolates with a P1 diameter of ≤25 mm were considered resistant) [8]. The Kirby–Bauer Pc disc diffusion zone of inhibition was virtually assessed as “sharp” or “fuzzy” [7]. A “sharp” edge at the inhibition zone around the disc suggested penicillinase production, whereas a “fuzzy” edge suggested no production. Both the zone diameter and appearance of the zone edge were recorded independently by two investigators. The cloverleaf test was also conducted to detect penicillinase [10]. Any deviation from a complete circle was considered positive for penicillinase. All isolates were stored at −80℃ until genetic analyses. The study protocol was examined and approved by the committee of the institution (Byotai-Seiri Laboratory).
After completing the phenotypic tests, all the stored isolates were sent to one laboratory (Kitasato Institute for Life Sciences) for further analyses. Two different primer sets of stau-blaZ-fwd/stau-blaZ-rev 488 were used to amplify blaZ by PCR from a mixture of 100–200 ng of extracted genomic DNA [10]. PCR by stau-blaZ-fwd/stau-blaZ-rev was selected as the standard, as there were no mutations in its primer sequences and the amplicon generated by this set was within the blaZ coding region. Amplification products (421-bp and 674-bp) were resolved on a 1.5% agarose gel, stained with ethidium bromide, and visualized using an ultraviolet transilluminator. We also confirmed the correct sequences of the amplicons of all positive isolates by PCR. ATCC 29213 (penicillinase-positive) and ATCC 25923 (penicillinase-negative) were used as quality control strains for the BMD method, phenotypic tests, and PCR methods [11].
Two hundred isolates were recovered from 13 sterile specimens (11 blood/1 joint fluid/1 pleural effusion) and 187 non-sterile specimens (120 respiratory tract-origin/52 skin-origin/14 urine/1 stool) of patients (109 men/91 women; median age 76 years, range 0–102 years). The relationship between phenotypic test data and blaZ PCR results is shown in Table 1. All isolates showed Pc MICs of ≤0.03 µg/mL based on the CLSI BMD method [7]. The isolates excluded from Table 1 showed negative results for the six phenotypic tests and two blaZ PCR methods. We observed only two blaZ-positive isolates (No. 28 and 33) with correct sequences, which were amplified by both primer sets stau-blaZ-fwd/stau-blaZ-rev and 486/488 (Table 1).
Sensitivity, specificity, and positive and negative predictive values of phenotypic tests using blaZ PCR as the standard are indicated in Table 2. We found a low sensitivity (50%) and positive predictive value (PPV, 50%) of the nitrocefin-based test, low PPV (18.2%) of the P10 zone edge test, low sensitivity (50%) of the P10 diffusion test, low PPV (50% and 22.2%) of the P2 zone edge test and P2 diffusion test, and low sensitivity (50%) of the cloverleaf test.
Table 3 summarizes the prevalence of blaZ among S. aureus isolates, various primer sets to amplify blaZ, and different amplicon sizes, used in previous studies and the current study [9101112131415]. blaZ prevalence in Japan (2.7%, 3.5%, and 1%) was lower than that observed in Germany, the United States, Australia, and Switzerland (14.2%, 9.5%, 24.2%, and 40.9%, respectively), although it remains unclear why the prevalence was low in Japan. The use of only one PCR primer set and lack of amplicon sequencing may have caused false-positive or false-negative results because of polymorphisms within the blaZ sequence (including the PCR primer sequence regions) [516] or targeting a genetic region peripheral to blaZ [10]. False-positive results are induced by non-functional mutant genes that are inadequately counted, and false-negative results occur when functional mutant genes are missed [11]. Therefore, we applied two primer sets for sequencing.
In conclusion, these data suggest the low performance (sensitivity and PPV) of the six phenotypic tests because of the low prevalence (1%) of blaZ in S. aureus isolates from Japan. The decreased sensitivities of the phenotypic tests in this study may be related to the use of a highly selective collection of isolates, all of which had low Pc MICs (≤0.03 µg/mL). Therefore, additional studies on isolates with borderline MICs (0.06 and 0.12 µg/mL) are needed.
Acknowledgements
This study was financially supported by a Grant-in-Aid for Clinical Research from the General Foundation Tokyo Hoken Kai (to Y. Takayama and T. Takahashi, 2015–2017). The authors wish to thank Ms. Haruno Yoshida (Laboratory of Infectious Diseases, Kitasato Institute for Life Sciences, Kitasato University) for her helpful assistance. In addition, we wish to thank Editage (www.editage.jp) for English language editing.
References
1. Zhang HZ, Hackbarth CJ, Chansky KM, Chambers HF. A proteolytic transmembrane signaling pathway and resistance to β-lactams in staphylococci. Science. 2001; 291:1962–1965. PMID: 11239156.
2. Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with β-lactam resistance in Staphylococcus aureus. J Bacteriol. 1984; 158:513–516. PMID: 6563036.
3. Lowy FD. Antimicrobial resistance: the example of Staphylococcus aureus. J Clin Invest. 2003; 111:1265–1273. PMID: 12727914.
4. Gregory PD, Lewis RA, Curnock SP, Dyke KG. Studies of the repressor (BlaI) of β-lactamase synthesis in Staphylococcus aureus. Mol Microbiol. 1997; 24:1025–1037. PMID: 9220009.
5. Olsen JE, Christensen H, Aarestrup FM. Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J Antimicrob Chemother. 2006; 57:450–460. PMID: 16449305.
6. Nannini EC, Stryjewski ME, Singh KV, Bourgogne A, Rude TH, Corey GR, et al. Inoculum effect with cefazolin among clinical isolates of methicillin-susceptible Staphylococcus aureus: frequency and possible cause of cefazolin treatment failure. Antimicrob Agents Chemother. 2009; 53:3437–3441. PMID: 19487449.
7. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing: 22nd informational supplement. Document M100-S22. Wayne, PA: CLSI;2012.
8. European Committee on Antimicrobial Susceptibility. Breakpoint tables for interpretation of MICs and zone diameters version 2.0. European Committee on Antimicrobial Susceptibility. Basel, Switzerland: 2012. http://www.eucast.org/eucast_susceptibility_testing/breakpoints/.
9. Kaase M, Lenga S, Friedrich S, Szabados F, Sakinc T, Kleine B, et al. Comparison of phenotypic methods for penicillinase detection in Staphylococcus aureus. Clin Microbiol Infect. 2008; 14:614–616. PMID: 18397333.
10. El Feghaly RE, Stamm JE, Fritz SA, Burnham CA. Presence of the blaZ beta-lactamase gene in isolates of Staphylococcus aureus that appear penicillin susceptible by conventional phenotypic methods. Diagn Microbiol Infect Dis. 2012; 74:388–393. PMID: 22959917.
11. Papanicolas LE, Bell JM, Bastian I. Performance of phenotypic tests for detection of penicillinase in Staphylococcus aureus isolates from Australia. J Clin Microbiol. 2014; 52:1136–1138. PMID: 24452169.
12. Sugimoto K, Komatsu M, Tanaka S, Tanimoto E, Kubo Y, Okada J, et al. Performance assessment of various β-lactamase production tests with penicillin-susceptible Staphylococcus aureus isolates. Nihon Rinsho Biseibutsugaku Zasshi. 2011; 21:136. (in Japanese).
13. Komatsu M. Interpretation of antimicrobial susceptibility testing data for Staphylococcus spp. Medical Technology. 2014; 42:84–87. Accessed on 4th October 2017. http://www.de-hon.ne.jp/digital/bin/product.asp?sku=6491008607014801500P in Japanese.
14. Tomei T, Kamichi Y, Maruo M, Aragaki M, Nakasone I. Comparison of tests for detecting penicillinase in Staphylococcus aureus. In : Proceeding of the 49th JAMT Kyushu-shibu Igakukensagakkai; 2014. p. p26. Accessed on 4th October 2017. http://rnavi.ndl.go.jp/books/2017/04/025818752.php in Japanese.
15. Hombach M, Weissert C, Senn MM, Zbinden R. Comparison of phenotypic methods for the detection of penicillinase in Staphylococcus aureus and proposal of a practical diagnostic approach. J Antimicrob Chemother. 2017; 72:1089–1093. PMID: 28069883.
16. Milheirico C, Portelinha A, Krippahl L, de Lencastre H, Oliveira DC. Evidence for a purifying selection acting on the β-lactamase locus in epidemic clones of methicillin-resistant Staphylococcus aureus. BMC Microbiol. 2011; 11:76. PMID: 21496235.
Table 1
Relationship between results of phenotypic penicillinase tests and blaZ PCR as the standard
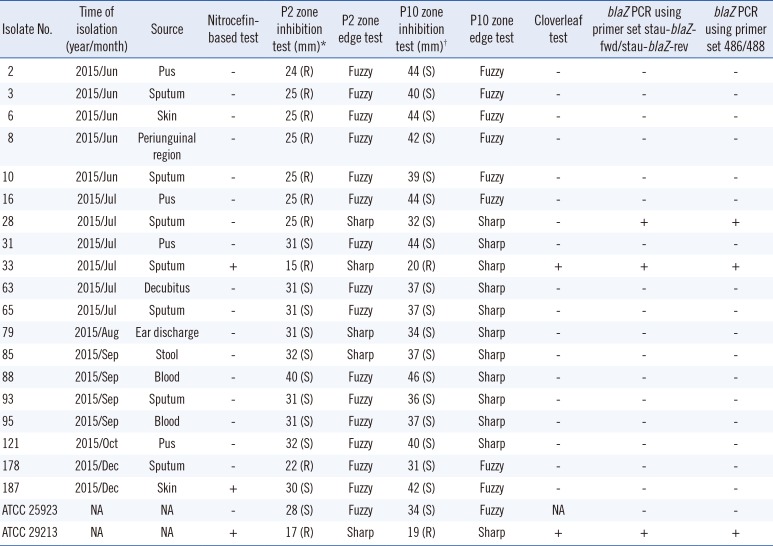
ATCC 25923 and ATCC 29213 were applied as penicillinase-negative and -positive controls, respectively.
*P2 zone diameters were interpreted according to the EUCAST criteria (isolates with P1 diameter of ≤25 mm were considered resistant) [11], and its interpretations are given in parentheses; †P10 zone diameters were interpreted according to the CLSI criteria (isolates with P10 diameter of ≤28 mm were considered resistant) [11], and its interpretations are given in parentheses.
Abbreviations: R, resistant; S, susceptible; NA, not applicable; +, positive; −, negative.
Table 2
Sensitivity, specificity, and positive and negative predictive values of penicillinase tests using blaZ PCR as the standard
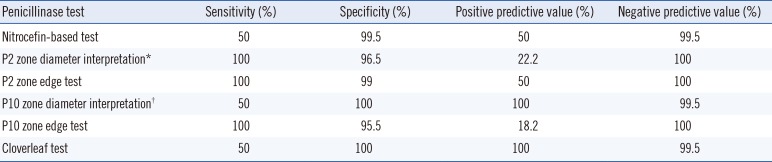
Table 3
Prevalence of blaZ among Staphylococcus aureus isolates in previous reports and the current study

| Year of report (reference) | Country of isolation (total N of collected isolates) | Percentage (%) of blaZ gene prevalence among all isolates | Primer set used to amplify blaZ | Amplicon size (bp) |
|---|---|---|---|---|
| 2008 [9] | Germany (197) | 14.2 | stau-blaZ-fwd/stau-blaZ-rev | 421 |
| 2011 [12, 13] | Japan (450) | 2.7 | stau-blaZ-fwd/stau-blaZ-rev | 421 |
| 2012 [10] | United States (105) | 9.5 | stau-blaZ-fwd/stau-blaZ-rev, 487/373, & 486/488 | 421, 377, & 674 |
| 2014 [11] | Australia (157) | 24.2 | blaZ-F/blaZ-R | 326 |
| 2014 [14] | Japan (170) | 3.5 | ND | ND |
| 2017 [15] | Switzerland (215) | 40.9 | blaZ-fwd/blaZ-rev & blaZ F1/blaZ R1 | 418 & 533 |
| This report | Japan (200) | 1 | stau-blaZ-fwd/stau-blaZ-rev & 486/488 | 421 & 674 |




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download