Abstract
Background
Fungi, especially Aspergillus flavus, can cause chronic rhinosinusitis with nasal polyposis and modulate host innate immune components. The objective of this study was to examine the serum levels of T helper (Th) cell subset Th1, Th2, and Th17 cytokines and total IgE in patients having chronic rhinosinusitis with nasal polyposis and Aspergillus flavus infection.
Methods
A case-control study including 40 patients with chronic rhinosinusitis with nasal polyposis and 20 healthy controls was conducted. Aspergillus flavus infection was confirmed by standard potassium hydroxide (KOH) testing, culture, and PCR. Serum samples of all patients and controls were analyzed for various cytokines (interleukins [IL]-1β, IL-2, IL-4, IL-6, IL-17, IL-21, IL-27, TGF-β) and total IgE by ELISA. Data from patients with Aspergillus flavus infection and healthy volunteers were compared using the independent t-test and non-parametric Mann-Whitney U test.
Results
Aspergillus flavus infection was found in 31 (77.5%) patients with chronic rhinosinusitis with nasal polyposis. IL-1β, IL-17, IL-21, and TGF-β serum levels were significantly higher in these patients than in controls; however, IL-2, IL-4, IL-6, and IL-27 levels were lower. Compared with nine (22.5%) patients without Aspergillus flavus infection, IL-17 level was higher while IL-2 level was lower in patients with Aspergillus flavus infection. Total IgE was significantly higher in patients with Aspergillus flavus infection than in controls.
Conclusions
High levels of IL-17 and its regulatory cytokines in patients with chronic rhinosinusitis with nasal polyposis infected by Aspergillus flavus raise a concern about effective disease management and therapeutic recovery. Surgical removal of the nasal polyp being the chief management option, the choice of post-operative drugs may differ in eosinophilic vs. non-eosinophilic nasal polyposis. The prognosis is likely poor, warranting extended care.
Inflammation of the nasal cavity and the contiguous paranasal sinus mucosa is termed chronic rhinosinusitis (CRS). CRS symptoms last for more than 12 weeks and may or may not be associated with nasal polyposis (NP), referred to as CRSwNP and CRSsNP, respectively [1]. NP, which is associated with inflammation of the nasal cavity and paranasal sinuses with persistent nasal discharge, anosmia, stuffy nose, and headache, is a devastating illness of unknown etiology [2]. The two forms of CRS have different underlying pathophysiological mechanisms, response to treatment modalities, and prognosis. NP forms an important social issue with high global incidence (2–5%) [3] and unsatisfactory treatment outcomes [4]. CRSwNP is further categorized as eosinophilic and non-eosinophilic based on the nasal lavage cytology pattern [5]. Eosinophils have been implicated in the formation of edematous polyps from sinuses to nasal cavity [1].
Several bacteria and fungi can cause CRS [3]. Fungi, especially Aspergillus flavus can cause severe acute or chronic sinusitis in immuno-competent hosts. In response to airborne fungi entrapped in the sinonasal mucus, the toxic granules released from eosinophils and mucosal inflammation cause CRS. Inflammatory cytokines play a role in controlling the fungal burden and reducing inflammation [67].
The pathophysiology of polyp formation through the recruitment and activation of inflammatory cells and their survival involve cytokines. Though both Th1- and Th2-type cytokines are involved, their roles are not yet fully understood. Coa et al [8] reported high expression of Th1, Th2, and Th17 in CRSwNP patients than in controls. Zhang et al [910] observed a non-eosinophilic Th1/Th17-biased response in patients with CRSwNP in an Asian population. Th17 cells, which release the pro-inflammatory cytokine IL-17; and T regulatory (Treg) cells, which secrete the anti-inflammatory cytokine IL-10 and transforming growth factor-β (TGF-β), are distinct from Th1 and Th2 T-cell subsets. Responding Th17 cells are considered vital in autoimmunity, inflammation, and allergic reactions [1112]. Moreover, IL-2 and TGF-β act as growth factors for Treg cells and promote their differentiation, while TGF-β along with IL-6 promotes the differentiation of IL-17 cells [13]. The inflammatory cells that infiltrate nasal polyps differ in Asian and Western patients; this is attributed to the different genetic predispositions and environmental conditions. Hence, an attempt to understand the underlying pathogenesis in an Indian population was undertaken. We investigated the serum levels of various cytokines (IL-1β, IL-2, IL-4, IL-6, IL-17, IL-21, IL-27, TGF-β) and immunoglobulin E (IgE) in CRSwNP patients with and without A. flavus infection and compared them with the levels in healthy controls. We attempted to correlate the findings to understand the underlying immunopathogenesis of the CRSwNP.
This prospective, analytical, case-control study was conducted at the University College of Medical Sciences (University of Delhi) and Guru Teg Bahadur Hospital, Delhi, India from January 2014 to February 2015. Subjects enrolled in the study included 40 immunocompetent patients (25 males and 15 females) between 17 and 65 years of age (mean age 29.26±15.92 years) clinically diagnosed as having CRSwNP and confirmed by experts through history, examination, and histopathological and laboratory investigations. The visual analog scale (VAS) was used to assess symptom scores [14]. The Lund and Mackay classification was used to grade preoperative computed tomography (CT) scans of nose and paranasal sinuses [15]. Patient age, sex, and duration of the disease were noted. Twenty age- and sex-matched adult healthy volunteers (11 males and nine females; mean age 27.4±4.18 years) without any history of allergy or any previous nasal or sinus surgery were enrolled as controls (Table 1).
The Institutional Ethical Committee-Human Research (IEC-HR) of the University College of Medical Sciences and Guru Teg Bahadur Hospital approved the study (approval dated 23/03/2012). Written informed consent was obtained from all patients and controls before enrollment and sample collection.
Ten percent xylocaine was sprayed into each nostril pre-operatively to increase the nasal lumen for collecting sufficient nasal lavage. Using a sterile syringe and needle, each nostril was flushed with 20 mL saline 2 minutes after xylocaine spray. Patients were asked to forcefully exhale in a sterile pan after taking a deep inspiration breath and holding it before injection of saline. Nasal lavage was also obtained from healthy volunteers. Post-operatively, tissue biopsy samples in normal saline and formalin from NP patients were obtained and transferred immediately to the laboratory for examinations. Tissue biopsies were subjected to direct KOH (10%) examination and cultured at 25℃ on Sabouraud dextrose agar (SDA) with antibiotics (0.4 g/L chloramphenicol, 0.04 g/L gentamycin). The rate of growth, surface texture, and pigmentation were noted. Standard tease mount using lactophenol cotton blue was prepared from the growth in culture for the identification of A. flavus [16]. Histopathological examination was done on tissue biopsies using hematoxylin and eosin/Gomori methenamine silver staining for fungal hyphae, eosinophils, neutrophils, Charcot–Leyden crystals, inflammatory cells, and for any evidence of tissue invasion.
Venous blood samples (4 mL each) were collected from all patients with CRSwNP and healthy volunteers in plain Vacutainer blood collection tubes, and serum was isolated by centrifugation at 3,000 rpm for 10 minutes. Sera were stored at –80℃ until analysis of cytokine and IgE levels.
DNA was purified from concentrated nasal lavage and tissue samples using the HiYield genomic DNA extraction kit (Real Biotech Corporation [RBC], Taipei, Taiwan) following the manufacturer's guidelines. The exonic region of the aspergillopepsin PEPO gene was amplified from the purified DNA using previously reported primers [17] specific for A. flavus (forward primer: 5′-CGACGTCTACAAGCCTTCTGGAAA-3′, reverse primer: 5′-CAGCAGACCGTCATTGTTCTTGTC-3′). PCR method as described previously [18] was conducted with some modifications in a Mastercycler Personal (Eppendorf). Briefly, the following thermal cycling parameters were used: 95℃ for 5 minutes; 40 cycles of 95℃ for 30 seconds, 58℃ for 30 seconds, and 72℃ for 45 seconds; and 72℃ for 10 minutes. Amplicons were visualized on agarose gel (2%) containing 0.5 mM ethidium bromide using an ultraviolet transilluminator.
Serum levels of the cytokines IL-1β, IL-2, IL-4, IL-6, IL-17, IL-21, IL-27, and TGF-β were estimated in duplicates using commercially available ELISA kits (Diaclone, Besançon, France). Total IgE levels were also estimated in duplicate using an ELISA kit from Calbiotech (El Cajon, CA, USA), following the manufacturer's protocol.
The independent t-test or non-parametric Mann–Whitney U test were used for comparing the serum levels of cytokines between patients with CRSwNP and healthy volunteers. Data were expressed as the mean±SD. The Shapiro Wilk test was used to check normal distribution of the data. All tests were two-sided, with the significance level set at P<0.05. All analyses were carried out using SPSS software version 20.0 (SPSS, Chicago, IL, USA).
Patient profiles, including age, sex, clinical details, and relevant laboratory investigations, are described in Table 1. Patients and healthy volunteers were compared in terms of age, male/female ratio, and the presence or absence of allergy. The mean age of CRSwNP patients was 29.26±15.92 years. The mean duration of the disease was 12.49±8.60 months, while the symptom and CT scores were recorded to be 13.07±1.78 and 8.63±1.60, respectively.
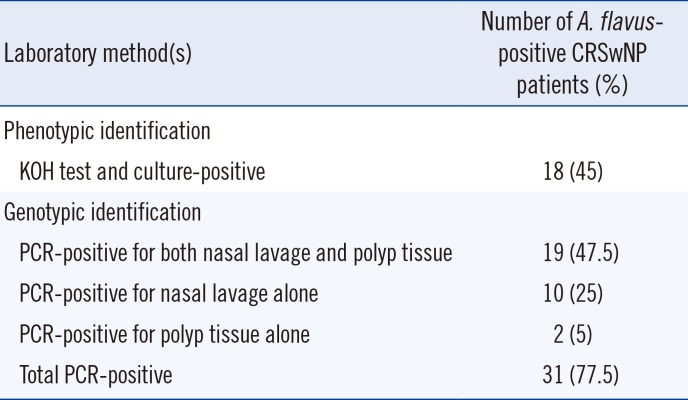
All patients with CRSwNP (n=40) were tested for A. flavus infection, and a total of 31 (77.5%) were found positive. Forty-five percent of CRSwNP patients were positive for A. flavus by KOH testing and culture. Based on PCR, A. flavus was detected in nasal lavage of 72.5% of patients, and in polyp tissue of 52.5% of patients (Table 2). Thus, we found A. flavus to be significantly associated with CRSwNP.
Out of the nine (22.5%) A. flavus-negative CRSwNP patients, we found Aspergillus fumigatus in two (5%), Aspergillus niger in one (2.5%), Alternaria spp. in one (2.5%), Curvularia spp. in one (2.5%), Rhizopus spp. in one (2.5%), and no microbial growth in three (7.5%) patients.
Based on the presence of inflammatory cells, 54.84% (17/31) of A. flavus-positive patients were found to be eosinophilic and 45.16% (14/31) neutrophilic. On the basis of the presence of eosinophils, mucin, allergy, and fungal stain, 41.93% (13/31) of A. flavus-positive patients were categorized as allergic fungal rhinosinusitis. Blood eosinophilia was considered increased in 26 out of 40 (65%) patients based on absolute eosinophil counts >300/mm3.
Serum levels of cytokines IL-2, IL-4, IL-6, and IL-27 were significantly lower in A. flavus-positive CRSwNP patients than in healthy controls (Fig. 1A). However, IL-1β, IL-17, IL-21, and TGF-β levels were significantly higher (Fig. 1B). Compared with A. flavus-negative CRSwNP patients, the IL-2 level was reduced (P=0.081), while IL-17 level was increased (P=0.061) in A. flavus-positive patients; this result was not statistically significant (data not shown).
Total IgE levels were significantly higher in A. flavus-positive patients than in controls (Fig. 2). IgE levels between A. flavus-positive and -negative patients was not different statistically (P=0.072; data not shown).
Various potential etiological factors, such as anatomical variants, microbial and, particularly, fungal infection and/or colonization, atopic response, intolerance to acetyl salicylic acid, and sometimes combinations of all these, have been implicated in the CRS process [1920]. The upregulation of inflammation of the nose, laterally, by these factors results in NP development [2]. The pathogenic process is known to begin as a result of dysregulation of the Th1/Th2 balance with excessive Th1 or Th2 activation. IL-17 has recently been implicated in regulating NP inflammation by attracting eosinophils and subsequent tissue reaction. Shen et al [21] have described atopy as an important factor in NP pathogenesis that may expand the disproportionation of Th17/Treg balance, thereby aggravating polyposis via the IL-17 inflammatory pathway. Additionally, CRSwNP is associated with high eosinophil levels, which distinguishes it from CRSsNP; however, in early stages of polyposis, the levels of IgE may not differ considerably from those in non-polypoid sinusitis [1].
Based on histopathological criteria, 41.93% of cases were categorized as allergic fungal rhinosinusitis, characterized by a fungal allergen-mediated IgE allergic response and associated with abundant accumulation of eosinophils in the mucin as described by Bent and Kuhn [22]. High total IgE can induce an allergic inflammatory cascade, involving the recruitment of eosinophils and basophils, leading to a series of cellular events [23]. Further, high specific IgE affects the underlying pathogenesis, leading to adverse prognosis and poor management of patients.
Fungal allergens have been shown to elicit IgE-mediated allergic and possibly, type III (immune complex)-mediated mucosal inflammation in atopic hosts [24]. In such sensitized individuals, fungi persist locally and stimulate destructive immune responses. Further, eosinophilic buildup in the mucin boosts inflammatory mediators in the extended sinuses. The association of high IL-1β and TGF-β with low IL-27, IL-6, and IL-2 indicate their involvement in Th17 subset differentiation in CRSwNP patients with A. flavus infection, suggesting Th17/Treg equilibrium as a key factor in modulating the immune responses and is perhaps crucial for disease progression in these patients [24]. Our findings are similar to previous findings in a Chinese population, which suggested that a skewed Th17 response with impaired Treg function is crucial both locally and systematically for NP pathogenesis [21]. However, the comparative IL-2 increase in CRSwNP patients without A. flavus infection may have suppressed the levels of IL-17, IL-1β, TGF-β, and associated cytokines.
It is important to understand that cytokines act sequentially, in concert or in conflict, in a given immune response, depending on whether the host–pathogen interaction is dominated by the conidial or the hyphal form of a fungus [25]. Repeated exposure to Aspergillus conidia induces the co-evolution of Th1, Th2, and Th17 responses in the nasal mucosa of patients with CRS. Low IL-2 level is a poor trigger for Treg cells and favors the predominance of Th17 subsets. The absence of cytokines IL-6 and IL-27 does not hinder Th cell differentiation mediated by IL-21 and IL-17 [23]. Our study showed that the low levels of IL-6 and IL-27 and high level of IL-1β might be responsible for Th17 cell proliferation; however, the role of IL-21 in inducing the production of IL-17 from naive T cells cannot be ignored [26]. Therefore, the differentiation of Th17 cells might involve the cooperation of IL-6 and/or IL-21 with TGF-β. This is supported by the study of Korn et al [26], which suggested that cooperation of IL-21, a member of the IL-2 cytokine family, with TGF-β induces the generation of Th17 cells from naïve IL-6-deficient T cells, and that T cells deficient in IL-21 receptor generate a defective Th17 response. We also observed a lethargic Th1 response, which counter balances persistently high IL-17 levels in patients with CRSwNP with A. flavus infection, leading to tissue damage. The low IL-4 level as indicated by our study correlates well with the suppressed Th2 response in CRSwNP as a whole.
Persistent fungal exposure highlights a role for IL-17 in upholding the inflammatory response in a majority of patients with CRSwNP with A. flavus infection. In our study, the predominant Th17/Th1 response favored an ongoing inflammatory process in patients with average disease duration of 8–10 months that abrogated the Th2 response. Hence, it is important to identify the sustained existence of an antigenic stimulus at the infection site, which provides a continuous inflammatory stimulus for developing a CD4-positive T-cell response. IgE level, eosinophils in the local tissue/biopsy, and peripheral eosinophilia are all hallmarks of a Th2 response, while low IL-4 and IL-6 are indicative of a dose-dependent adaptive response that has been overtaken by a chronic inflammatory response under the influence of IL-17 [27].
In the absence of a strong Th1 response, Tregs facilitate an antigen-specific Th17-mediated response in the presence of various cytokines and inhibit fungal clearance. Because Tregs ultimately have a role in fungal clearance, their expansion is necessary to limit the disease. Like Treg cells, proliferation of the Th17 response is stimulated by fungal cell-wall components via dectin-1 interaction [28]. Additionally, Th17 differentiation can be facilitated by Tregs, and Tregs themselves can convert to Th17 cells [29] in a process assisted by dendritic cells [30]. This may drive the initial Th17 response aided by the diminished Th1 response, since Th1 and Th2 cytokines inhibit Th17 development. Murdock et al [27] observed that Th17 cell levels increased and acquire a dominant adaptive response on repeated exposure to A. fumigatus conidia, while the Th2 cell levels remained stable or were reduced. The time-dependency of IL-17 function and its role in exacerbating and/or diminishing Th2 inflammatory responses have been shown in several studies. Thus, as an alternative immunological pathway to repeated low fungal allergen exposures, a Th17 response might arise.
In conclusion, the role of IL-17 in driving CRSwNP in A. flavus-positive patients, with either eosinophilic or neutrophilic inflammation promoted by high TGF-β, was revealed. However, whether the role of cytokines in instructing CD4-positive T-cell differentiation is fungi-specific or not needs to be elucidated for conclusively identifying IL-17 as an important marker for disease progression. Whether the role of raised IgE in causing mast-cell activation and inflammation is independent of the type of stimulus or allergen, which requires further investigation, raises concern regarding the effective, appropriate, and targeted therapeutic intervention in allergic disorders like CRS and associated relapses.
Acknowledgement
This work was financially supported by the grant from the Department of Biotechnology (DBT), Government of India, New Delhi [No. BT/PR5191/MED/29/447/2012].
References
1. Polzehl D, Moeller P, Riechelmann H, Perner S. Distinct features of chronic rhinosinusitis with and without nasal polyps. Allergy. 2006; 61:1275–1279. PMID: 17002702.
2. Bachert C, Wagenmann M, Rudack C, Hopken K, Hiltebrandt M, Wang D, et al. The role of cytokines in infectious sinusitis and nasal polyposis. Allergy. 1998; 53:2–13. PMID: 9491223.
3. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004; 114:155–212. PMID: 15577865.
4. Wynn R. Recurrence rates after endoscopic sinus surgery for massive sinus polyposis. Laryngoscope. 2004; 114:811–813. PMID: 15126735.
5. Soler ZM, Sauer DA, Mace J, Smith TL. Relationship between clinical measures and histopathologic findings in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2009; 141:454–461. PMID: 19786212.
6. Zelante T, De Luca A, Bonifazi P, Montagnoli C, Bozza S, Moretti S, et al. IL-23 and the Th17 path way promote inflammation and impair antifungal immune resistance. Eur J Immunol. 2007; 37:2695–2706. PMID: 17899546.
7. Bozza S, Zelante T, Moretti S, Bonifazi P, DeLuca A, D’Angelo C, et al. Lack of toll IL-1R8 exacerbates Th17 cell responses in fungal infection. J Immunol. 2008; 180:4022–4031. PMID: 18322211.
8. Cao PP, Li HB, Wang BF, Wang SB, You XJ, Cui YH, et al. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009; 124:478–484. PMID: 19541359.
9. Zhang N, Holtappels G, Claeys C, Huang G, van Cauwenberge P, Bachert C. Pattern of inflammation and impact of Staphylococcus aureus enterotoxins in nasal polyps from southern China. Am J Rhinol. 2006; 20:445–450.
10. Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. JAllergy Clin Immunol. 2008; 122:961–968. PMID: 18804271.
11. Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007; 8:345–350. PMID: 17375096.
12. Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005; 6:1133–1141. PMID: 16200068.
13. Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, et al. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007; 26:371–381. PMID: 17363300.
14. Lund VJ, Holmstrom M, Scadding GK. Functional endoscopic sinus surgery in the management of chronic rhino sinusitis. An objective assessment. J Laryngol Otol. 1991; 105:832–835. PMID: 1753193.
15. Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997; 117:S35–S40. PMID: 9334786.
16. Leck A. Preparation of Lactophenol Cotton Blue Slide Mounts. Community Eye Health. 1999; 12:24.
17. Logotheti M, Kotsovili-Tseleni A, Arsenis G, Legakis NI. Multiplex PCR for the Discrimination of A. fumigatus, A. flavus, A. niger and A. terreus. J Microbiol Methods. 2009; 76:209–211. PMID: 18992777.
18. Mazumdar A, Das S, Saha R, Ramachandran VG, Gupta N, Sharma S, et al. Correlation of nucleic acid amplification based detection and conventional methods of identification of Aspergillus flavus species in chronic rhinosinusitis. Research & Reviews: J Microbiol Biotechnol. 2013; 2:61–66.
19. Ricchetti A, Landis BN, Maffioli A, Giger R, Zeng C, Lacroix JS. Effect of anti-fungal nasal lavage with amphotericin B on nasal polyposis. J Laryngol Otol. 2002; 116:261–263. PMID: 11945184.
20. Garín L, Armengot M, Alba JR, Carda C. Correlations between clinical and histological aspects in nasal polyposis. Acta Otorrinolaringol Esp. 2008; 59:315–320. PMID: 18817712.
21. Shen Y, Tang XY, Yang YC, Ke X, Kou W, Pan CK, et al. Impaired balance of Th17/Treg in patients with nasal polyposis. Scand J Immunol. 2011; 74:176–185. PMID: 21375554.
22. Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994; 111:580–588. PMID: 7970796.
23. Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope. 2000; 110:799–813. PMID: 10807359.
24. Chakrabarti A, Denning DW, Ferguson BJ, Ponikau J, Buzina W, Kita H, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope. 2009; 119:1809–1818. PMID: 19544383.
25. Bozza S, Gaziano >R, Spreca A, Bacci A, Montagnoli C, di Francesco P, et al. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J Immunol. 2002; 168:1362–1371. PMID: 11801677.
26. Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, et al. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007; 448:484–487. PMID: 17581588.
27. Murdock BJ, Shreiner AB, McDonald RA, Osterholzer JJ, White ES, Toews GB, et al. Co-evolution of TH1, TH2, and TH17 responses during repeated pulmonary exposure to Aspergillus fumigatus conidia. Infect Immun. 2011; 79:125–135. PMID: 21041495.
28. Van Beelen AJ, Zelinkova Z, Taanman-Kueter EW, Muller FJ, Hommes DW, Zaat SA, et al. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007; 27:660–669. PMID: 17919942.
29. Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, et al. Reprogrammed FoxP3+ T regulatory cells become IL-17 antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008; 181:3137–3147. PMID: 18713984.
30. Osorio F, Leibund Gut-Landmann S, Lochner M, Lahl K, Sparwasser T, Eberl G, et al. DC activated via dectin-1 convert Treg into IL-17 producers. Eur J Immunol. 2008; 38:3274–3281. PMID: 19039774.
Fig. 1
Serum levels of various cytokines in healthy controls and patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) and A. flavus infection. The bars in (A) show the levels of downregulated cytokines (IL-2, IL-4, IL-6, and IL-27), and those in (B) show the levels of upregulated cytokines (IL-17, IL-21, IL-1β, and TGF-β), compared with healthy controls. *P<0.05; **P<0.01; ***P<0.001.
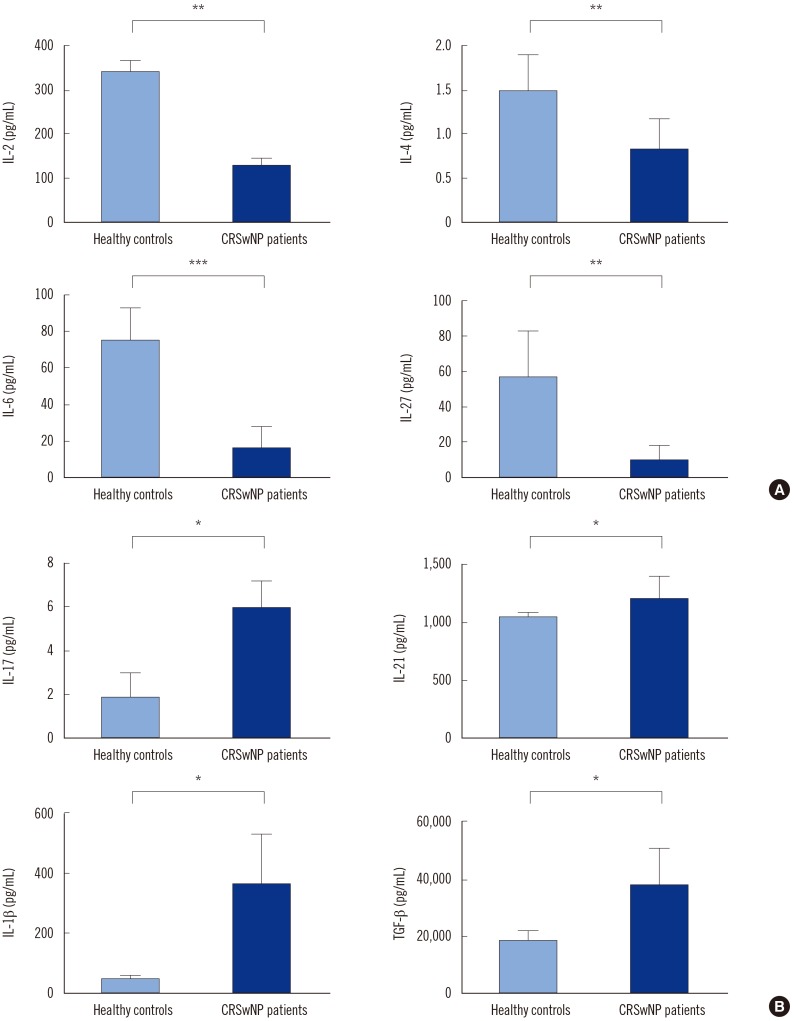
Fig. 2
Serum IgE levels in healthy controls and patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) and A. flavus infection.
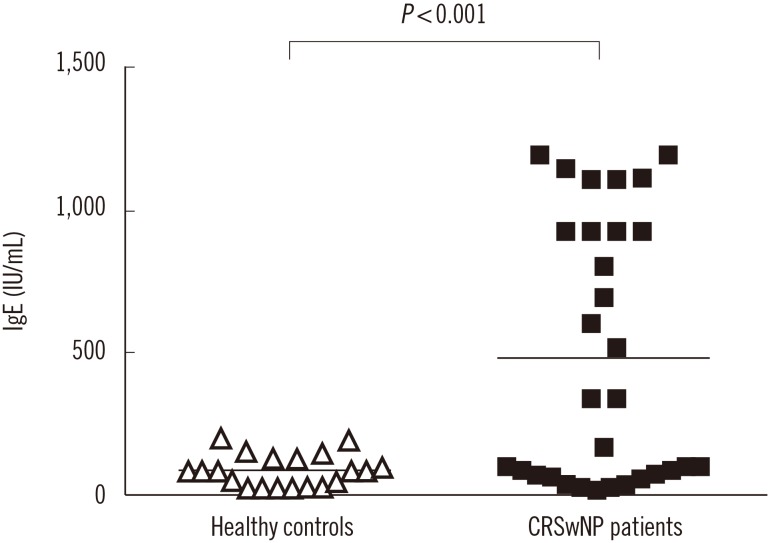
Table 1
Characteristics of patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) and healthy controls
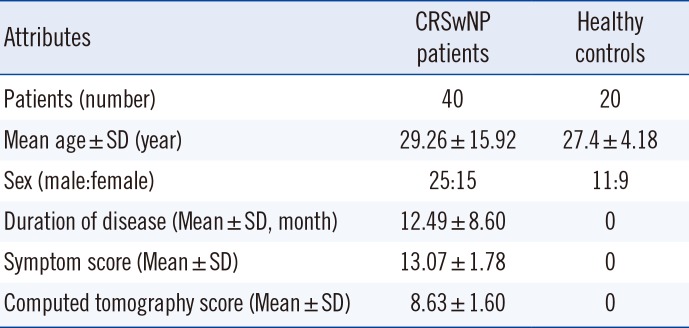
Table 2
Detection of A. flavus in 40 patients with chronic rhinosinusitis with nasal polyposis (CRSwNP) by different laboratory methods




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download