Dear Editor,
Mastocytosis refers to a group of rare clinical disorders, in which mast cells expand abnormally and accumulate in various organs [1 2]. Mast cell leukemia (MCL) is a rare and aggressive form of systemic mastocytosis and accounts for 1% of all mastocytosis [3]. Due to its rarity, making a prompt diagnosis is difficult when unaware of the morphological characters of the abnormal mast cells. The aim of this study is to aid the correct diagnosis of MCL, especially when the mast cells are in immature form.
MCL is diagnosed when bone marrow (BM) biopsy indicates the infiltration of immature mast cells and BM aspiration smear shows ≥20% atypical mast cells. A leukemic variant of MCL is when there are ≥10% circulating mast cells; when this criterion is not met, MCL is categorized as the aleukemic variant [1]. Since MCL was first described in 1906, no treatment has yet been established [456].
A 71-yr-old woman presenting with a 1-month history of epigastric pain was initially referred to the gastroenterology department. Her complete blood cell count showed bicytopenia (hemoglobin level, 94 g/L; leukocyte count, 6.71×109/L; platelet count, 40×109/L). Abdominopelvic computed tomography revealed many small lymph nodes at the periportal area and mild hepatosplenomegaly. Persistent bicytopenia and leukoerythroblastic reaction on the peripheral blood smear led us to perform a BM biopsy.
BM aspirate smears showed approximately 67% of hypogranulated and degranulated bizarre-looking cells, mostly with heavy cytoplasmic vacuoles, and showing the characteristic features of surface projections with eccentrically positioned oval nuclei and hypogranular cytoplasm with focal granule accumulations with or without granule fusion. Cells with bilobed and polylobed nuclei with high to low nucleus-to-cytoplasm ratio were frequently observed (Fig. 1). These cells were later recognized to be atypical promastocytes [7].
Myeloperoxidase and periodic acid-Schiff staining of the cells was negative. Flow cytometric immunophenotyping showed strong positive expressions of CD13 (98.38%), CD33 (97.43%), and CD117 (83.32%) but lacked MPO, CD34, HLA-DR, CD 41, CD14, and B- and T-lymphoid lineage markers. One day after the BM biopsy was performed, the patient rapidly developed leukocytosis (leukocyte count, 20.32×109/L), and thrombocytopenia was aggravated (platelet count, 23×109/L). Her condition deteriorated rapidly with hepatic failure and progressive metabolic acidosis, and the patient eventually died of multiorgan failure.
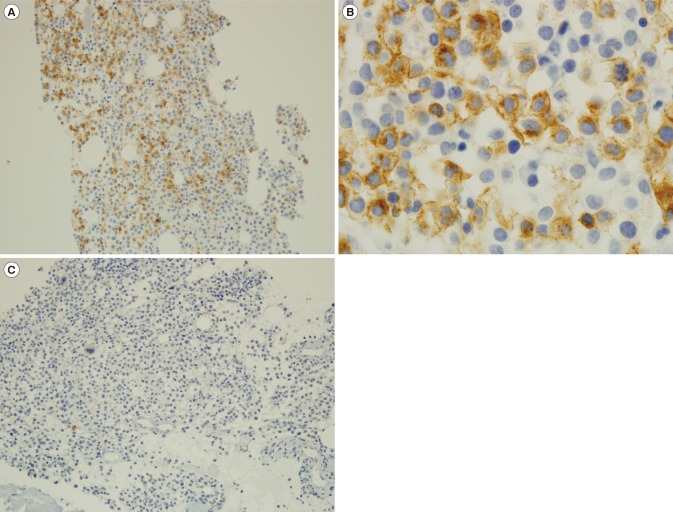
BM trephine biopsy revealed infiltration of immature-looking cells, which were positive for CD2 (Fig. 2A, B) and negative for CD25 (Fig. 2C) through immunohistochemical staining. Serum tryptase level was >200 µg/L (normal range: <11.0 µg/L). KIT mutation screening with PCR of D816V showed no mutation, and further direct sequencing of exons 9 and 11 was wild type. Based on these results, the final patient diagnosis was a de novo aleukemic variant of MCL.
The main challenge of diagnosing this rare disease is the lack of suspicion of MCL owing to its heterogeneous and unspecific clinical presentations. In this case, the typical morphologic features of mature mast cells were not seen in the BM and circulating mast cells were completely absent in the peripheral blood, making the diagnosis difficult. Most of the abnormal mast cells were composed of premature promastocytes with marked morphologic atypia, which have been shown to be the predominant cell type in the aggressive type of systemic mastocytosis and related to poor prognosis of the disease, as in the present patient [7].
CD2 is commonly expressed in MCL and could be helpful in recognizing abnormal mast cells [8]. A reliable diagnostic factor of MCL, the elevated serum tryptase level in this patient (>200 µg/L) provided a valuable clue for the diagnosis of MCL, but an exact quantitative evaluation was not performed [9 10]. Previous data focusing on MCL showed that nearly 50% of de novo MCL patients had the KIT D816V mutation. Our patient did not show the KIT D816V mutation with wild-type sequences in exons 9 and 11 as another distinct feature; however, it is difficult to exclude other possible mutations in different exons of the KIT gene at this point.
In conclusion, we present a case of a de novo aleukemic variant of MCL with highly aggressive disease progression. We suggest possible risk factors like the disease being de novo, the absence of mature mast cells in the BM, and elevated serum tryptase level. Recognizing the characteristic morphology of MCL remains a critical step for guiding diagnosis in the right direction, as early diagnosis may give the patient a chance to undergo early treatment. Further, new treatment combinations such as high-dose steroid therapy, KIT target therapy, chemotherapy, and he-matopoietic stem cell transplantation should be investigated in the future. An established standard therapy is expected to improve the prognosis of this fatal disease.
References
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC;2008. p. 54–63.
2. Valent P, Akin C, Escribano L, Födinger M, Hartmann K, Brockow K, et al. Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. Eur J Clin Invest. 2007; 37:435–453. PMID: 17537151.
3. Georgin-Lavialle S, Lhermitte L, Dubreuil P, Chandesris MO, Hermine O, Damaj G. Mast cell leukemia. Blood. 2013; 121:1285–1295. PMID: 23243287.
4. Valentini CG, Rondoni M, Pogliani EM, Van Lint MT, Cattaneo C, Marbello L, et al. Mast cell leukemia: a report of ten cases. Ann Hematol. 2008; 87:505–508. PMID: 18172645.
5. Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009; 113:5727–5736. PMID: 19363219.
6. Noack F, Sotlar K, Notter M, Thiel E, Valent P, Horny HP. Aleukemic mast cell leukemia with abnormal immunophenotype and c-kit mutation D816V. Leuk Lymphoma. 2004; 45:2295–2302. PMID: 15512820.
7. Sperr WR, Escribano L, Jordan JH, Schernthaner GH, Kundi M, Horny HP, et al. Morphologic properties of neoplastic mast cells: delineation of stages of maturation and implication for cytological grading of mastocytosis. Leuk Res. 2001; 25:529–536. PMID: 11377677.
8. Baumgartner C, Sonneck K, Krauth MT, Kneidinger M, Födinger M, Hauswirth AW, et al. Immunohistochemical assessment of CD25 is equally sensitive and diagnostic in mastocytosis compared to flow cytometry. Eur J Clin Invest. 2008; 38:326–335. PMID: 18363719.
9. Savini P, Rondoni M, Poletti G, Lanzi A, Quercia O, Soverini S, et al. Serum total tryptase level confirms itself as a more reliable marker of mast cells burden in mast cell leukaemia (aleukaemic variant). Case Rep Hematol. 2015; 2015:737302. PMID: 25755899.
10. Theoharides TC, Valent P, Akin C. Mast Cells, Mastocytosis, and Related Disorders. N Engl J Med. 2015; 373:163–172. PMID: 26154789.
Fig. 1
Peripheral blood and bone marrow aspiration smear findings. (A) Peripheral blood findings show leukoerythroblastic reaction and no circulating mast cells (Wright-Giemsa stain, ×400). (B) Bone marrow aspiration finding shows atypical promastocytes, which are hypogranulated and degranulated bizarre-looking cells mostly with heavy cytoplasmic vacuoles. Characteristic features of surface projections with eccentrically positioned oval nuclei and hypogranulated cytoplasm, with focal granule accumulations with or without granule fusion are seen. Cells with bilobed and polylobed nuclei with high to low nucleus-to-cytoplasm ratio are frequently observed (Wright-Giemsa stain, ×1,000).

Fig. 2
Immunohistochemical staining of CD2 and CD25, which are used as markers for immature mast cells. (A) Im-munohistochemical staining shows positive CD2 staining. Bone marrow section showing multifocal and dense infiltrates of mast cells (×200). (B) High-power view of CD2 cytoplasmic staining of mast cells (×1,000). (C) Immunohistochemical staining shows negative CD25 staining (×200).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download