This article has been corrected. See "Erratum: Novel 4-bp Intronic Deletion (c.1560+3_1560+6del) in LEMD3 in a Korean Patient With Osteopoikilosis" in Volume 39 on page 235.
Abstract
Osteopoikilosis is an autosomal dominant bone disorder characterized by symmetric multiple osteosclerotic lesions throughout the axial and appendicular skeleton. Pathogenic variants in the LEMD3 have been identified as the cause of osteopoikilosis. LEMD3 encodes an inner nuclear membrane protein that interacts with bone morphogenetic protein (BMP) and transforming growth factor (TGF)-β pathways. We report the case of a 19-year-old man presenting with lower back pain and sciatica. His radiograph revealed bilateral and symmetrical multiple osteosclerotic bone lesions in both scapular areas. Sanger sequencing of LEMD3 revealed a four-base-pair deletion in intron 2 (c.1560+3_1560+6del), which was inherited from his father. We found that this four-base-pair deletion in intron 2 causes aberrant splicing and consequent deletion of exon 2. To the best of our knowledge, this is the first report of genetically confirmed osteopoikilosis in Korea.
Osteopoikilosis, also known as osteopathia condensans disseminata, is a rare and benign autosomal dominant disease characterized by symmetric but unequal distribution of osteosclerotic bone dysplasia in different parts of the skeleton. The diagnosis is often made on the basis of incidental radiologic findings with multiple small, sclerotic foci at the ends of the long bones, pelvis, sacrum, and bones of the hands and feet. Although it is usually asymptomatic, 15-20% of patients may have joint pain and effusion [1234].
Although the pathogenesis of osteopoikilosis has not been fully elucidated, heterozygous loss-of-function variants in the LEM domain-containing protein 3 (LEMD3) gene have been identified as the cause of osteopoikilosis by genome-wide linkage analysis in affected families [5]. In Korea, several cases of familial osteopoikilosis have been reported; however, all of these cases were diagnosed by radiological findings and clinical manifestations such as pelvic pain and lower abdominal discomfort, without genetic analysis of LEMD3 [6789]. We report a Korean patient with osteopoikilosis carrying a novel splice site variant in the LEMD3 gene.
A 19-yr-old man with lower back pain and sciatica presented to our hospital to rule out bone metabolic disease. Radiographs showed multiple osteosclerotic bone lesions in both scapular areas (Fig. 1A). He had a treatment history for short stature, and at the time he first visited our hospital, his height was 162 cm. Other than short stature, there were no remarkable findings in the connective tissue or skin. The proband's father also suffered from multiple joint pain, and the father's radiographs showed multiple, small, roundish radio-dense lesions in both proximal femurs and the iliac bone (Fig. 1B).
After obtaining informed consents, whole blood samples were collected from the patient and his father to analyze their LEMD3 status. Genomic DNA was extracted from peripheral blood leukocytes, and all 13 exons were amplified by PCR using an ABI 3730xl analyzer (Applied Biosystems, Foster City, CA, USA) and the BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) with primers designed by the authors. LEMD3 sequences were analyzed by using Sequencher software (Gene Codes Corp., Ann Arbor, MI, USA) and compared with the reference sequence (NM_014319.4).
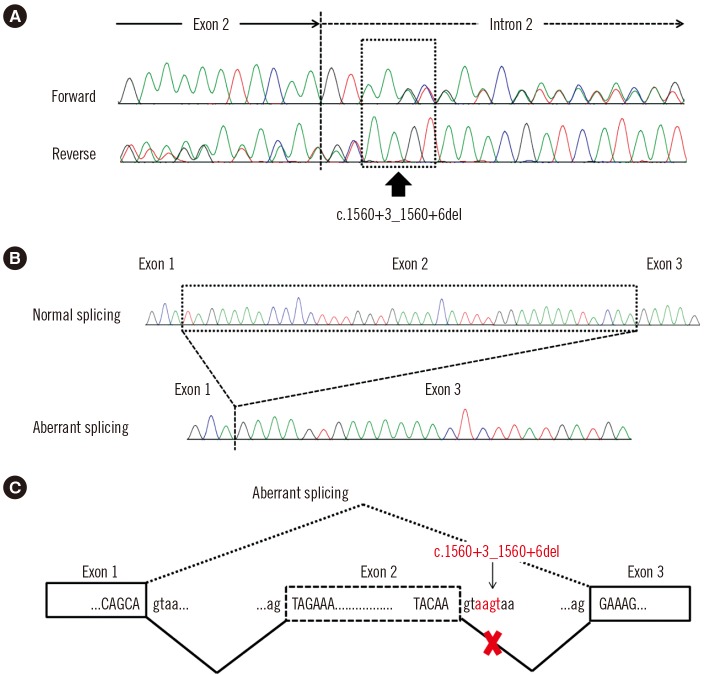
A four-base-pair deletion (c.1560+3_1560+6del) in intron 2 of LEMD3 was identified in the patient and his father (Fig. 2A). This variant has not been reported previously and was absent from the Single Nucleotide Polymorphism database (https://www.ncbi.nlm.nih.gov/snp), the Exome Aggregation Consortium (ExAC) database (http://exac.broadinstitute.org/), and the Korean Reference Genome database (http://152.99.75.168/KRGDB/). Reverse-transcription PCR (RT-PCR) and amplicon cloning results confirmed aberrant splicing. As expected, one clone with a deletion in exon 2 (r.1523_1560del) was identified (Fig. 2B and C), resulting in a frameshift in the LEMD3 protein (p.Ile508Argfs*3). On the basis of these results, we regard this variant as a pathogenic variant that may cause osteopoikilosis according to the standards and guidelines of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology [10].
Osteopoikilosis is an autosomal dominant bone disorder characterized by symmetric multiple osteosclerotic lesions throughout the axial and appendicular skeleton. Prevalence has been estimated to be 1/50,000 and occurs at the same rate in men and women [1]. Heterozygous loss-of-function variants in LEMD3 have been identified as the major cause of both osteopoikilosis and Buschke-Ollendorff syndrome (BOS, OMIM 166700), with or without melorheostosis [5]. BOS is a rare autosomal dominant disorder characterized by early-onset skin features, such as multiple cutaneous elastic hamartomas, and also osteopoikilosis [11]. To date, many variants of LEMD3 have been found, including nonsense, frame-shift, and splice site defects resulting in exon skipping, as was found in this case [12]. However, no phenotype-specific variants have been found in the LEMD3 gene [13].
LEMD3 is an inner nuclear membrane protein that interacts with both bone morphogenetic protein (BMP) and transforming growth factor (TGF)-β signaling pathways through interactions with Smad family proteins such as Smad1 (BMP-specific) and Smad2 (TGF-β specific). When LEMD3 is deactivated, it cannot downregulate Smad1 activation resulting in the promotion of bone formation via BMP signaling [5]. Due to the antagonistic effect of LEMD3 on TGF-β signaling, increased signaling in the TGF-β pathway can cause skin lesions in patients with BOS [14]. Therefore, loss-of-function variants in LEMD3 could lead to the abnormal growth of connective and bone tissue via enhanced BMP and TGF-β signaling [15].
In conclusion, we identified a novel splice site variant of LEMD3 causing osteopoikilosis in a Korean family. Our finding reinforces the idea that defects in the LEMD3 gene are associated with osteosclerotic bone dysplasia. To the best of our knowledge, this is the first case of osteopoikilosis in Korea where diagnosis was confirmed by genetic analysis.
References
1. Korkmaz MF, Elli M, Özkan MB, Bilgici MC, Dağdemir A, Korkmaz M, et al. Osteopoikilosis: report of a familial case and review of the literature. Rheumatol Int. 2015; 35:921–924. PMID: 25352085.
2. Paraskevas G, Raikos A, Stavrakas M, Spanidou S, Papaziogas B. Osteopoikilosis: a case report of a symptomatic patient. J Radiol Case Rep. 2009; 3:38–43. PMID: 22470634.
3. Benli IT, Akalin S, Boysan E, Mumcu EF, Kiş M, Türkoğlu D. Epidemiological, clinical and radiological aspects of osteopoikilosis. J Bone Joint Surg Br. 1992; 74:504–506. PMID: 1624505.
4. Debeer P, Pykels E, Lammens J, Devriendt K, Fryns JP. Melorheostosis in a family with autosomal dominant osteopoikilosis: report of a third family. Am J Med Genet A. 2003; 119A:188–193. PMID: 12749062.
5. Hellemans J, Preobrazhenska O, Willaert A, Debeer P, Verdonk PC, Costa T, et al. Loss-of-function mutations in LEMD3 result in osteopoikilosis, Buschke-Ollendorff syndrome and melorheostosis. Nat Genet. 2004; 36:1213–1218. PMID: 15489854.
6. Kim Y, Kim YI, Moon MK. Osteopoikilosis - Report of 6 cases. J Korean Orthop Assoc. 1978; 13:433–438.
7. Yune SH, Lee JK, Ahn SR, Rha SY, Park CH. A case report of familial osteopoikilosis. J Korean Orthop Assoc. 1986; 21:1133–1136.
8. Jun S, Kim YK, Kim IJ, Nam HY, Kim BS. Innumerable small bony nodular sclerotic lesions with negative findings on both bone scintigraphy and F-18 FDG PET: Osteopoikilosis in a patient of breast cancer. Nucl Med Mol Imaging. 2008; 42:256–258.
9. Yoo JH, Park YW, Park JS, Rowe KC, Chung KJ, Kim HK, et al. Non-familial osteopoikilosis around the both hip joints: a case report. J Korean Hip Soc. 2010; 22:86–89.
10. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–424. PMID: 25741868.
11. Reid EM, Baker BL, Stees MA, Stone SP. Buschke-Ollendorff syndrome: a 32-month-old boy with elastomas and craniosynostosis. Pediatr Dermatol. 2008; 25:349–351. PMID: 18577041.
12. Zhang Y, Castori M, Ferranti G, Paradisi M, Wordsworth BP. Novel and recurrent germline LEMD3 mutations causing Buschke-Ollendorff syndrome and osteopoikilosis but not isolated melorheostosis. Clin Genet. 2009; 75:556–561. PMID: 19438932.
13. Zhang Q, Mo ZH, Dong CS, Yang F, Xie YH, Jin P. Identification of a novel LEMD3 Y871X mutation in a three-generation family with osteopoikilosis and review of the literature. J Endocrinol Invest. 2016; 39:679–685. PMID: 26694706.
14. Lin F, Morrison JM, Wu W, Worman HJ. MAN1, an integral protein of the inner nuclear membrane, binds Smad2 and Smad3 and antagonizes transforming growth factor-beta signaling. Hum Mol Genet. 2005; 14:437–445. PMID: 15601644.
15. Hershkovitz D, Amitai B, Sprecher E. Familial cutaneous collagenomas resulting from a novel mutation in LEMD3. Br J Dermatol. 2007; 156:375–377. PMID: 17223882.
Fig. 1
Representative radiologic findings of the patients. (A) Ill-defined osteosclerotic lesions in both humoral head and scapula are observed in the proband. (B) Pelvis radiograph of the proband's father shows small, roundish radio-dense lesions in both proximal femurs and the iliac bone.
Abbreviations: Lt, Left; Rt, Right.
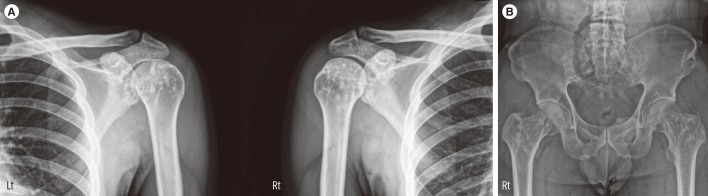
Fig. 2
Novel splice site variant in the LEMD3 gene. (A) Sequencing pattern of LEMD3 shows overlapping peaks due to a heterozygous variant in intron 2 (c.1560+3_1560+6del; arrow). (B) Cloning of reverse transcription (RT)-PCR products reveals two clones: a normal clone and an abnormal clone without exon 2. (C) Schematic illustration of aberrant splicing due to the heterozygous 4-bp deletion.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download